| Written RevisionQuestions | Multiple ChoiceRevision Questions | |
Summary Notes (Lesmahagow High School) |  |
For Higher Chemistry we will consider three properties of atoms and examine and explain how they change as we move across and down the periodic table. The video below discusses the reasons underlying the changes in the properties of the elements as we move down a group.
Explanation of trends moving down a group of the periodic table
Explanation of trends moving across a period (period 3) of the periodic table.
Atomic Size
The size of an atom is usually considered to be the covalent radius of the element. This is half the distance between the nuclei of two bonded atoms.
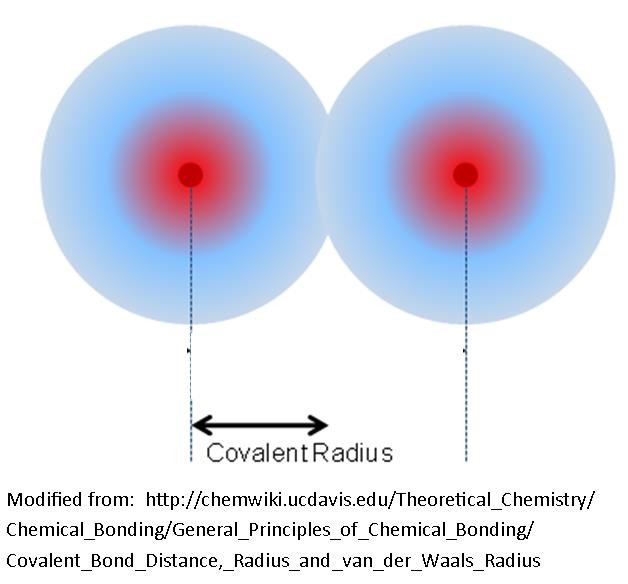 Electronegativity
Electronegativity
The reactivity of the elements in a given group will vary as we move down the group. The video below discusses the reasons why the
