| Written RevisionQuestions | Multiple ChoiceRevision Questions | |
Summary Notes (Lesmahagow High School) |  |
In manufacturing processes chemical reactions which progress too slowly (rates which are too low) will not be profitable (not econmically viable) and those with rates which are too high may become dangerously hot or even explosive. In either case, it is important for chemists to have an understanding of the factors which control the rate of a chemical reaction and be able to measure the rate of a chemical reaction.
Collision Theory
All chemical reactions involve atoms, molecules or ions colliding with each other.
The video below explains how collision theory explains how chemical reactions coccur.
Collision theory states that for reactions to occur:
- reacting particles must collide
- reacting particles must collide with sufficient energy
- reacting particles must collide with the correct alignment (collision geometry)
Factors affecting the rate of Reaction
The effect of temperature, concentration, pressure, surface area and collision geometry on the rate of chemical reactions can all be explained by considering collision theory.
Concentration of Reactants
(applies to reactions involving solutions only)
As the concentration of reactants increases, the rate of reactions also increases.
This can be easily explained by remembering that reactions only occur when reacting particles collide. If we squeeze more reacting particles into a smaller volume (increasing the concentration), then there is a greater chance that a collision will occur. More collisions means more reactions.
Pressure
(applies to reactions involving gases only)
The pressure of a gas is related to the number of molecules contained within a specific volume. The pressure increases when we squeeze the same number of molecules into a smaller volume.
As pressure increases, the rate of a chemical reaction increases.
This observation is also easily explained by remembering that reactions only occur when reacting particles collide. If we squeeze more reacting particles into a smaller volume (increasing the pressure), then there is a greater chance that a collision will occur.
More collisions means more reactions.
Surface Area
(applies to reactions involving solids)
The greater the surface area (smaller particle size), the faster the rate of reaction.
Solids can only react on their surface, the molecules under the surface are hidden, so cannot react. By breaking a larger lump into smaller pieces, a greater surface area is exposed, so there are more reacting particles, on the surface, able to take part in collisions.
More collisions means more reactions.
Temperature
The higher the temperature, the faster the rate of reaction.
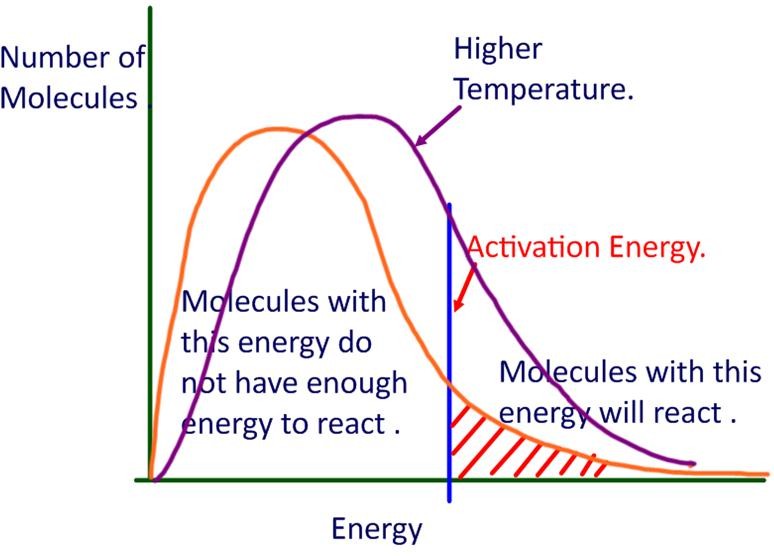
Temperature measures the average kinetic energy of the reacting particles in the substance. The graph below shows the energy of each molecule in a sample at higher and lower temperatures. The molecules have different levels of kinetic energy.
According to collision theory, molecules need to collide with sufficient energy before they can react. The minimum energy required to react is called the activation energy for the reaction.
Examining the graph, only some of the particles will have sufficient enough kinetic energy to react, and those with lower kinetic energy will not be able to react. As the temperature increases, the proportion of particles with higher energy is also increased.
If more molecules have sufficient energy to react, a greater of proportion of collisions will result in a successful reaction (fruitful collisions).
Collision Geometry
For a collision to cause a reaction, moleules must collide with the correct orientation. This is best illustrated by two examples:
In the reaction between ethene and hydrogen chloride, illustrated below, only if the hydrogen side of the H-Cl bond meets the carbon-carbon (double bond), will a reaction occur. If it collides with the ethene molecule in a different alignment no reaction will occur.
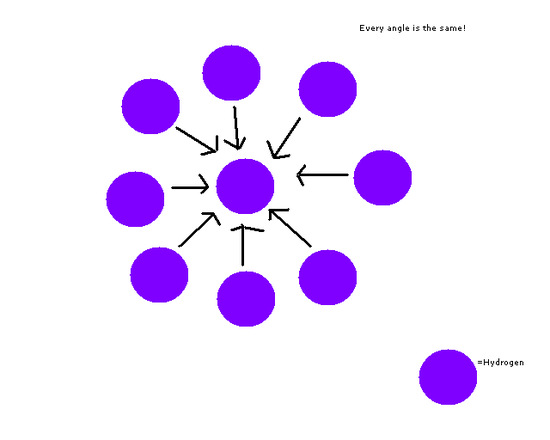
In this second example two hydrogen atom react to form a hydrogen molecule. No bonds are broken, only formed. In this case, because hydrogen atoms are symmmetrical in 3 dimensions collision geometry is ideintical regardless of where the atoms approach each other, therefore in this case, collision geometry has no impact on reaction rate – this is an unusual case.
Use of a Catalyst
A catalyst reduces the activation energy of a chemical reaction. It is also said to provide an alternative pathway for the reaction to occur.