| Written RevisionQuestions | Multiple ChoiceRevision Questions | |
Van der Waal’s Forces
All molecular elements and compounds and monatomic elements will condense and freeze at sufficiently low temperatures. For this to occur, some attractive forces must exist between the molecules or discrete atoms. Any “intermolecular” forces acting between molecules are known as van der Waals’ forces.
The image below accesses an animation which describes the form and properties of the various types of intermolecular forces.
There are several different types of van der Waals’ forces such as London dispersion forces and permanent dipole-permanent dipole interactions which includes hydrogen bonding.
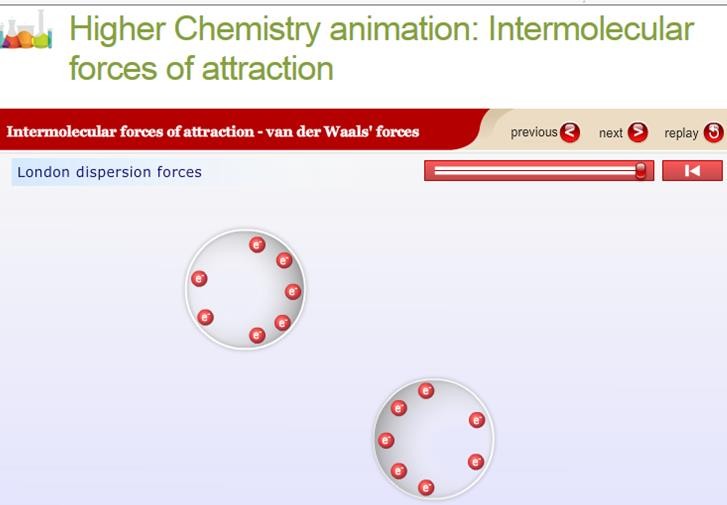
London Dispersion Forces
London dispersion forces are forces of attraction which can operate between all atoms and molecules. These forces are much weaker than all other types of bonding. They are caused by movement of electrons in atoms and molecules and form as a result of electrostatic attraction between temporary dipoles and induced dipoles . The strength of London dispersion forces is related to the number of electrons within an atom or molecule.
