| Written RevisionQuestions | Multiple ChoiceRevision Questions | |
Summary Notes (Lesmahagow High School) |  |
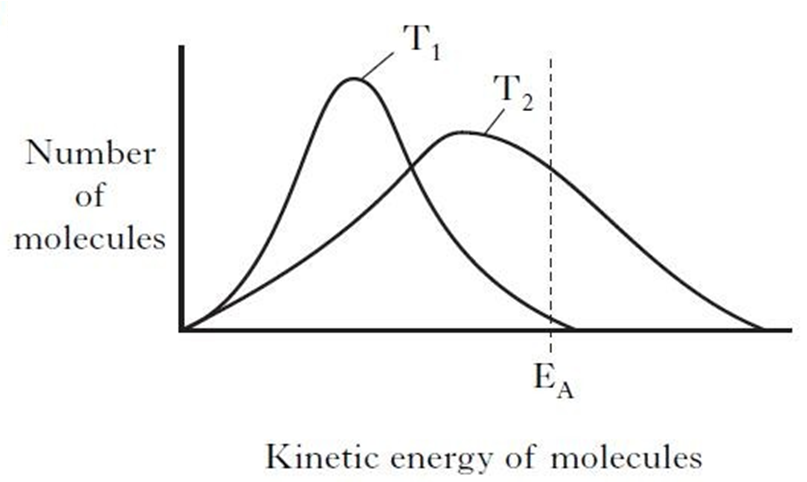
The particles (atoms or molecules) of solids liquids and gases are constantly in motion. The velocity with which a particle moves is a measure of its kinetic energy. The faster the speed, the greater the kinetic energy. For any substance, the particles making up the substance will be have a range of kinetic energy. For a collision between reactants, they must possess a minimum level of kinetic energy, called the activation energy (EA).
The kinetic energy of the particles can be shown on the graph below.
For a given reaction a line can be added showing the activation energy (EA on the graph above). Those particles with kinetic energy greater than the activation energy have the potential to react if a collision occurs. (Not all collisions result in a reaction as the colliding particles must also collide with favourable collision geometry) As we increase the temperature from T1 (lower) to T2 (higher), the proportion of molecules with sufficient energy to react increases. As a result, the rate of a reaction is increased when the temperature of the reaction is increased. It should be noted that the Activation Energy (EA) itself is unchanged, simply that the number of reactant particles which possess enough energy has increased.
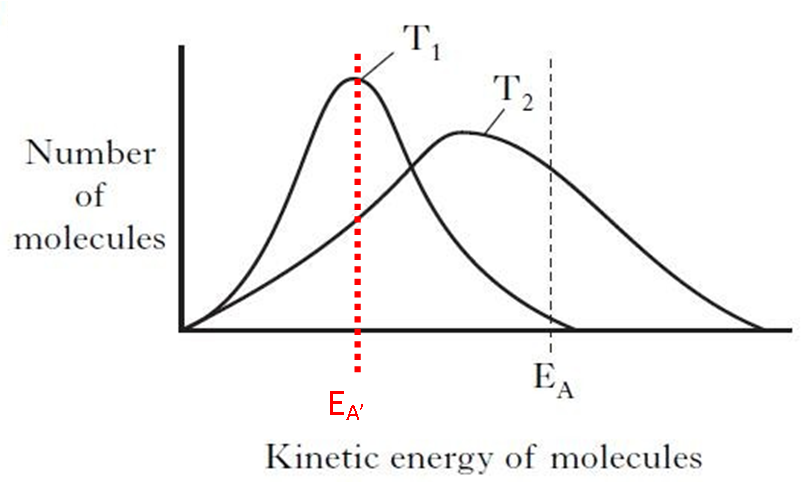
Use of a catalyst in a chemical reaction will reduce the Activation Energy (EA) for that reaction as the catalyst provides an alternative pathway for the reaction to occur. On that occasion, the graph can be redrawn to reflect the change in Activation Energy (EA).
It is clear from the graph that when the catalyst is added, causing a reduction in the Activation Energy (EA’), the proportion of reactant particles at temperature T1 (lower) now with sufficient energy to react has been greatly increased. As a result the catalyst will cause an increase in the rate of reaction wiothoout the need to increase the temperature. Obviously this will help reduce the cost of any industrial chemical process. Only through the use of a catalyst can the Activation Energy (EA) be changed.
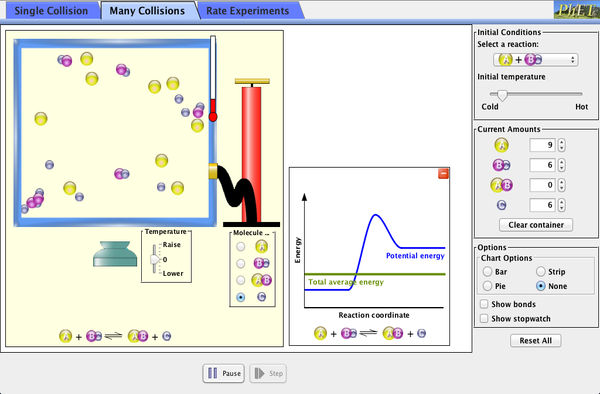
The simulator accessed by clicking the image below simulates a chemical reaction. Variables, inlcuding temperature can be altered to view their impact on reaction rate. The reaction profile included in the data usefully demonstrates the impact of altering temperature on the average kinetic energy of the reacting particles.