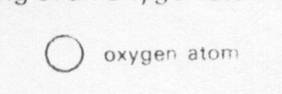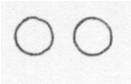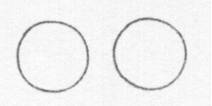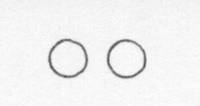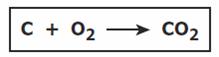| 1. 1 pts. |
In a class, a student accidentally poured sulphur into a jar of iron filings. She decided it would be easiest to separate the two using a magnet. This method would work because | |
| A. | The elements have formed a compound and so are not joined to each other | |
| B. | The elements have formed a mixture and so are joined to each other | |
| C. | The elerments have formed a mixture and so are not joined to each other | |
| D. | The elements have formed a compound and are joined to each other | |
| 2. 1 pts. |
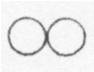
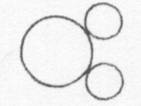
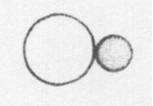
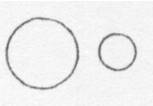
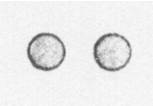
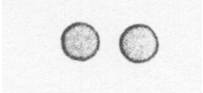
Below is a drawing of an oxygen atom. Which of the diagrams could represent a drawing of a molecule of oxygen?
|
||
| A. |
|
||
| B. |
|
||
| C. |
|
||
| D. |
|
||
| 3. 1 pts. |
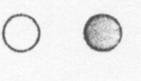
Iron is an element. Which of these could be a drawing of two iron atoms? | ||
| A. |
|
||
| B. |
|
||
| C. |
|
||
| D. |
|
||
| 4. 1 pts. |
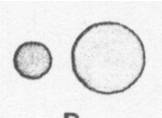
Carbon and zinc are elements. Which of the following could be a drawing of a carbon atom and a zinc atom? | ||
| A. |
|
||
| B. |
|
||
| C. |
|
||
| D. |
|
||
| 5. 1 pts. |
When the elements iron and sulphur are joined together a compound forms. This compound is | |
| A. | identical to iron | |
| B. | identical to sulphur | |
| C. | not like iron or sulphur | |
| D. | a mixture of lumps of iron and sulphur | |
| 6. 1 pts. |
What is used to test for hydrogen gas? | |
| A. | pH paper | |
| B. | lime water | |
| C. | a burning splint | |
| D. | a glowing splint | |
| 7. 1 pts. |
Which of these is a chemical reaction? | |
| A. | A lake freezes solid. | |
| B. | Gravel, sand, and water are mixed | |
| C. | A copper bar is rolled into a flat sheet | |
| D. | Vinegar bubbles when baking soda is added. | |
| 8. 1 pts. |
According to this equation, what happened to the carbon and oxygen?
|
||
| A. | They combined chemically to form a new compound. | ||
| B. | They combined chemically to form carbon and oxygen. | ||
| C. | They combined physically to form a new mixture. | ||
| D. | They combined physically to form a new element. | ||
| 9. 1 pts. |
Which process is a physical change? | |
| A. | Rusting iron | |
| B. | Burning coal | |
| C. | Tarnishing silver | |
| D. | Melting ice | |
| 10. 1 pts. |
Because zinc can combine with other substances but cannot be changed into a simpler substance by an ordinary chemical process, zinc is classified as — | |
| A. | a compound | |
| B. | a mixture | |
| C. | an element | |
| D. | an acid | |
| 11. 1 pts. |
A mixture of iron filings and sulphur can easily be separated by — | |
| A. | placing the mixture in water | |
| B. | performing a chemical reaction | |
| C. | heating the mixture | |
| D. | using a magnet | |
| 12. 1 pts. |
Which of these is best classified as a mixture? | |
| A. | Carbon dioxide | |
| B. | Water | |
| C. | Soil | |
| D. | Iron | |
| 13. 1 pts. |
If you break a piece of glass, the shape of the glass changes, but the properties in the fragments remain the same. Which of the following has occurred? | |
| A. | A chemical change | |
| B. | A temperature change | |
| C. | A state change | |
| D. | A physical change | |
| 14. 1 pts. |
A substance made up of two or more elements that have been chemically combined is called — | |
| A. | an atom | |
| B. | a compound | |
| C. | an element | |
| D. | a mixture | |
| 15. 1 pts. |
In an experiment combining vinegar and baking soda, gas is given off. In this chemical reaction, the vinegar and baking soda are — | |
| A. | reactants | |
| B. | products | |
| C. | elements | |
| D. | suspensions | |