| . 1 pts. |
From the list below select which is a compound. You may need to use your periodic table to help you decide. | |
| A. | Aluminium | |
| B. | Carbon Dioxide | |
| C. | Copper | |
| D. | Hydrogen | |
| 2. 1 pts. |
What forms when two, or more, elements join together? | |
| A. | mixture | |
| B. | element | |
| C. | compound | |
| D. | liquid | |
| 3. 1 pts. |
Using the periodic table you have in your homework diary find the symbol for the element gold. (if you don’t have your diary find a periodic table on the internet) | |
| A. | Ag | |
| B. | Au | |
| C. | Hg | |
| D. | au | |
| 4. 1 pts. |
Most elements in the periodic table are | |
| A. | metals | |
| B. | non-metals | |
| C. | compounds | |
| D. | man-made | |
| 5. 1 pts. |
What element is represented by the symbol Ag. Use the periodic table you have in your homework diary. (if you don’t have your diary find a periodic table on the internet) | |
| A. | Silver | |
| B. | Gold | |
| C. | Mercury | |
| D. | Arsenic | |
| 6. 1 pts. |
Approximately how many compounds are there? | |
| A. | Only a few | |
| B. | thousands | |
| C. | around a hundred | |
| D. | millions | |
| You can choose one, or more than answer for this question. | ||
| 7. 1 pts. |
Decide which of the following are physical changes? (Choose all that Apply) | |
| A. | Water Boiling | |
| B. | Striking a match | |
| C. | Iron rusting | |
| D. | Adding alka seltzer to water | |
| E. | petrol combusting in a car angine | |
| F. | A puddle disappearing in the sun | |
| G. | Ice melts | |
| 8. 1 pts. |
Decide which of the following are chemical changes (Choose all that Apply) | |
| A. | Frying an egg | |
| B. | Water boiling | |
| C. | Iron rusting | |
| D. | Striking a match | |
| E. | Ice melting | |
| F. | A puddle disappearing in the sun | |
| 9. 1 pts. |
Select the elements from the list below (Choose all that Apply) | |
| A. | Copper sulphate | |
| B. | Iron | |
| C. | Sugar | |
| D. | Water | |
| E. | Hydrogen | |
| F. | Carbon dixoide | |
| G. | Flour | |
| H. | Gold | |
| I. | Brass | |
| J. | Silver | |
| 10. 1 pts. |
Select the compounds from the list below. (Choose all that Apply) | |
| A. | Copper sulphate | |
| B. | Iron | |
| C. | Silver | |
| D. | Flour | |
| E. | Water | |
| F. | Carbon dixoide | |
| G. | Gold | |
| H. | Brass | |
| I. | Sugar | |
| J. | Iron | |
| 11. 1 pts. |
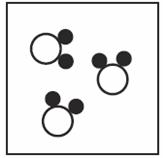
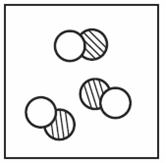
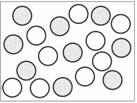
Which of the diagrams below show a compund? (Choose all that Apply) | ||
| A. |
|
||
| B. |
|
||
| C. |
|
||
| D. |
|
||
| 12. 1 pts. |
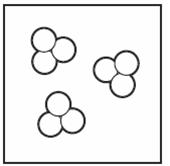
Which of the diagrams below show an element? (Choose all that Apply) | ||
| A. |
|
||
| B. |
|
||
| C. |
|
||
| D. |
|
||
| 13. 1 pts. |
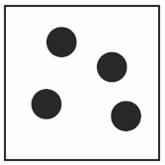
Which of the following shows a mixture? | ||
| A. |
|
||
| B. |
|
||
| C. |
|
||
| D. |
|
||
| 14. 1 pts. |
In her chemistry class, Jemima added a spatula of a white compound into a colourless solution. This caused the solution to fizz and the white powder disappeared. She was left with a colourless solution. How could Jemima tell that a chemical reaction had occurred? | |
| A. | A colour change had occurred | |
| B. | A gas was given off | |
| C. | The test tuibe had heated up | |
| D. | A solid was formed | |
| 15. 1 pts. |
When a chemical reaction takes place…. | |
| A. | Solutions always turn cloudy | |
| B. | A gas is always given off | |
| C. | A colour change is always seen | |
| D. | A new substance is always produced | |