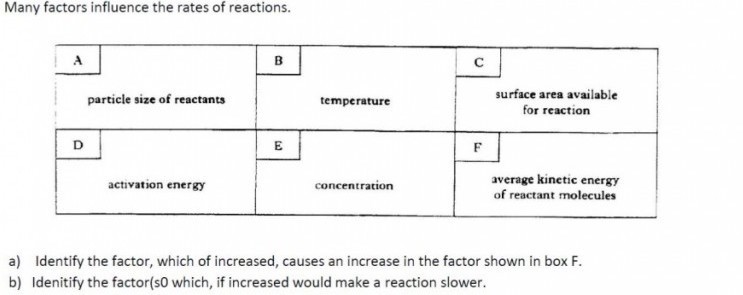
 |
 You should now be familiar with the factors which affect the rate of a chemical reaction. You should now be familiar with the factors which affect the rate of a chemical reaction.
|
Higher Chemistry Unit 1 Consolidation Exercises
Hyndland Chemistry Department
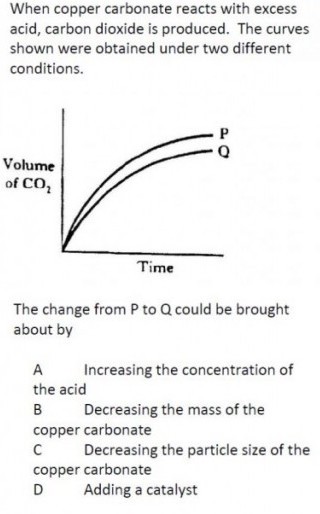 |
For this question, it is important that you notice the differences between the two lines, P & Q. The two differences are the height which the reaction reaches (i.e. the maximum product produced), and the rate at which the reaction proceeds.
Moving from P to Q the rate has reduced. This would suggest that changes in variables which slow reactions would lead to the change. You can find more on factors affecting the rate of reaction here. This consideration is sufficient to answer the question, however, you should also ponder that the lower maximum volume of CO2 is also explained by the correct choice. |
| Jill | Smith |
| Eve | Jackson |
Ions form when electrons are completely lost or gained by atoms. Pauling devised the comncept of ionic character in which a bond with 100% ionic character has no sharing of electrons and a bond with 0% ioninc character would have electrons equally shared. The degree to which sharing can occur is determined by the relative electronegativity of the atoms involved in the bond.
Welcome to blogs.glowscotland.org.uk – Glasgow. This is your first post. Edit or delete it, then start blogging!
Glow Blogs uses cookies to enhance your experience on our service. By using this service or closing this message you consent to our use of those cookies. Please read our Cookie Policy.