British Science Week: Experiment Time
5/6, along with P2/3 explored osmosis through experimentation.
Firstly the pupils enjoyed learning about solids, liquids, and gasses where they made a human solid, human liquid, and human gas.
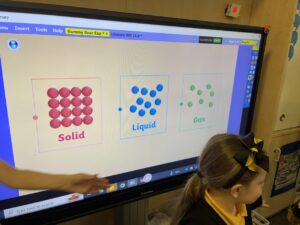
Solids consists of lots of tiny particles packed close together:
Liquids consist of less tiny particles which are spread out:
Gasses consist of the least tiny particles which are spread out even more:
Experiment time:
P5/6 created hypotheses of what might happen when we placed a solid (gummy bear) into a liquid (water) and left it overnight.
P2/3 led the learning by showing P5/6 what to do.
We left the cups containing our solid and liquids overnight to investigate today. When we returned the gummy bears has gotten bigger. We learned that this was due to a process called osmosis where water molecules transfer from a region of high water concentration to a region of low water concentration.
Finally, we discussed how we could use this knowledge to remove the water from the gummy bear. Some hypotheses we have put to the test are;
- placing the gummy bear in a solution of water and lots of salt
- placing the gummy bear in a solution of water and lots of sugar
- placing the gummy bear in just salt
- placing the gummy bear in just sugar
Why don’t you ask your child about the final outcome?



















