Revision Questions |
A | B | C | D | E |
Calculating the mass of one mole (calculating gfm) |
 |
 |
 |
 |
 |
Calculating the mass of fractions of a mole. |
|||||
Calculating the number of moles in a given mass. |
| |
 |
In these revision exercises you are asked to use the relationship between number of moles, gram formula mass and the mass of a substance to work out the answer. You should be familiar with the triangle formula and how to manipulate it. All answers have been calculated based on the RAM values detailed in the SQA National 5 databook, published in 2021. |
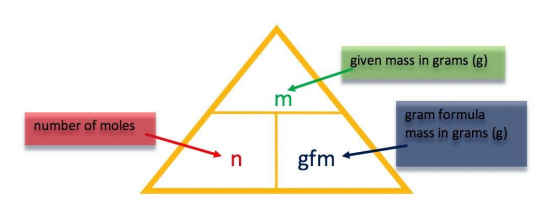 |
Question 1:
Calculate the mass of one mole of the following compounds:
| a) | CaO | b) | KCl | c) | NaBr |
| |
|
|
|||
| d) | LiCl | e) | Fe2O3 | f) | PbBr4 |
| |
|
|
|||
| g) | h) | i) | |||
| |
|
|
|||
| j) | k) | l) | |||
| |
|
|
|||
| m) | n) | o) | |||
| |
|
|
Question 2
Find the mass of each of the following:
| a) | 0.2 moles of: | b) | 0.3 moles of: | |
c) | 0.25 moles of: | ||
| |
|
|
||||||
| d) | 3 moles of: | e) | 10 moles of: | f) | 0.4 moles of: | |||
| |
|
|
||||||
| g) | 12 moles of: | h) | 2 moles of: | i) | 0.5 moles of: | |||
| j) | 0.8 moles of: | k) | 1.5 moles of: | l) | 2.4 moles of: | |||
Question 3
Find the mass of each of the following:
Question 4:
Calculate the number of moles in each of the following masses of compounds:
Question 5
Calculate the number of moles in the masses of the following compounds:

