Isomers are hydrocarbons which have the same molecular formula, but a different structural formula. This means they have the same numbers and types of atom, but they are arranged differently. Isomers may have different physical and chemical properties
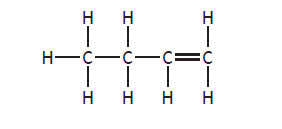
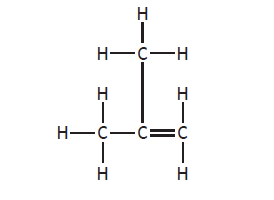
When two hydrocarbon structures differ, with one having branch(es) the second being a straight chain hydrocarbon (e.g. but-1-ene (C4H8) and methylpropene (C4H8)), they are isomers)
 |
 |
but-1-ene
|
methylpropene
|
It is worth noting that alkenes and cycloalkanes, because they both have the same general formula (CnH2n), will always be isomers when they have the same molecular formula (e.g. cyclobutane (C4H8) and butene (C4H8) are isomers).
 |
 |
cyclobutane
|
but-1-ene
|
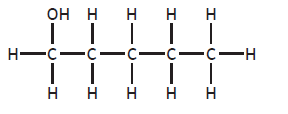
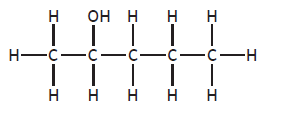
Finally, isomers also occur when the position of any functional group is altered. (e.g. butan-1-ol (C4H9OH) and butan-2-ol (C4H9OH) are isomers).
 |
 |
pentan-1-ol
|
pentan-2-ol
|
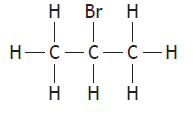 |
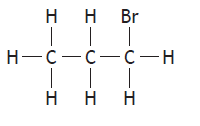 |
1-bromopropane
|
2-bromopropane
|
You must be careful not to confuse structures drawn in different directions or where the main chain is drawn bent. These are not isomers, as if you name them, you will find they are the same structural formula
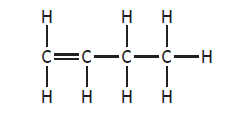 |
 |
but-1-ene
|
but-1-ene
|
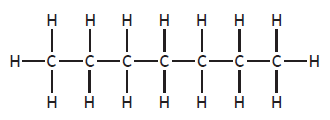 |
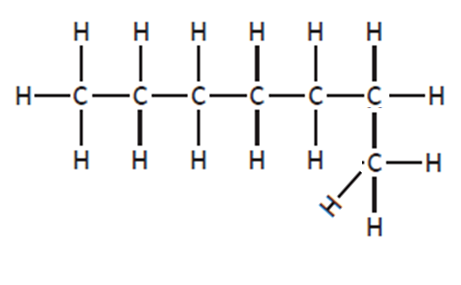 |
heptane
|
heptane
|













