| Identifying Chemical & Physical Change | (I) | (II) | (III) |
 |
 |
 |
SUMMARYChanges of substances can be classified as physical or chemical. The key difference between the two is that for a chemical change a new substance is ALWAYS produced. Signs of a chemical reaction:
For physical change a change of state or shape occurs, but crucially, no new substance is formed.
* Ice, water and steam (or vapour) are chemically the same compound, H2O, but just in solid, liquid or gas state. As ice melts or water boils, it is still H2O. The liquid water can be refrozen to form ice. |
| 1. | |||
| GEN 2011/12 | |||
| 2. | |||
| GEN 2012/5 | |||
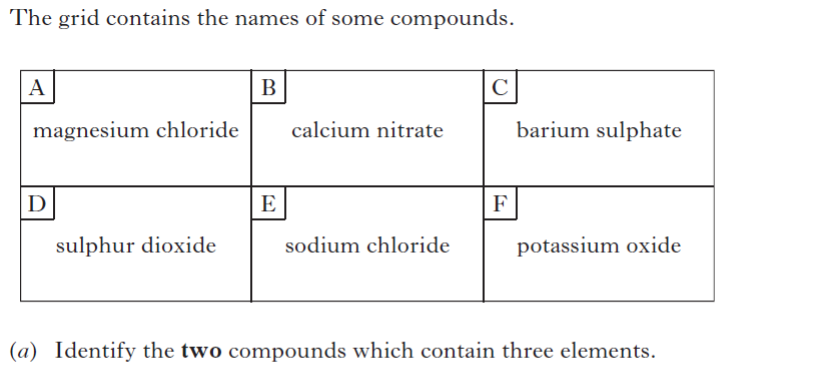
| 3. | |||
 |
|||
| GEN 2013/4 | |||
| 4. | |||
 |
|||
| GEN 2007/4 | |||
| 5. | |||
 |
|||
| GEN 2010/11 | |||
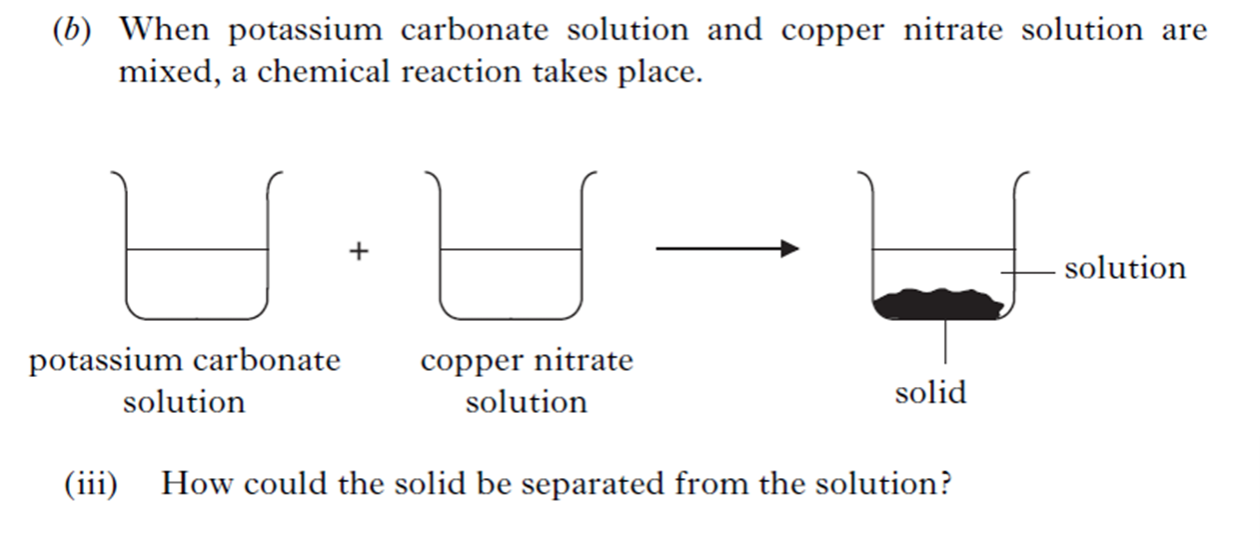
| 6. | |||
 |
|||
 |
|||
| GEN 2010/13 | |||
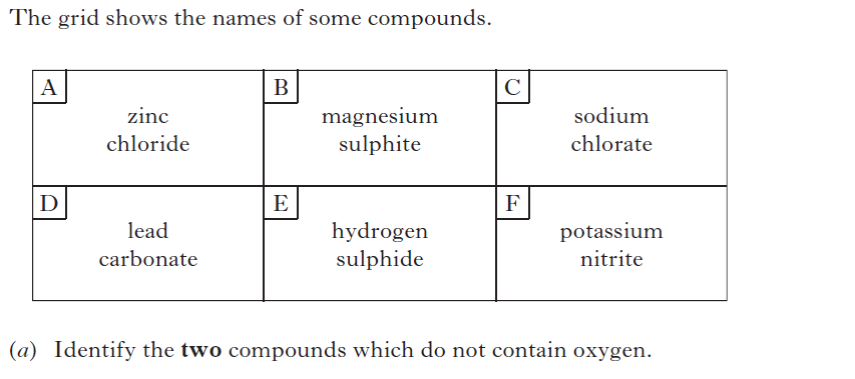
| 7. | |||
 |
|||
| GEN 2012/6 | |||
| 8. | |||
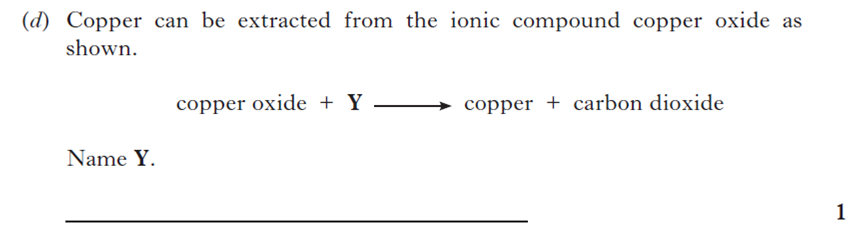 |
|||
| GEN 2012/18 | |||
| 9. | |||
 |
|||
 |
|||
| GEN 2013/16 | |||
| 10. | |||
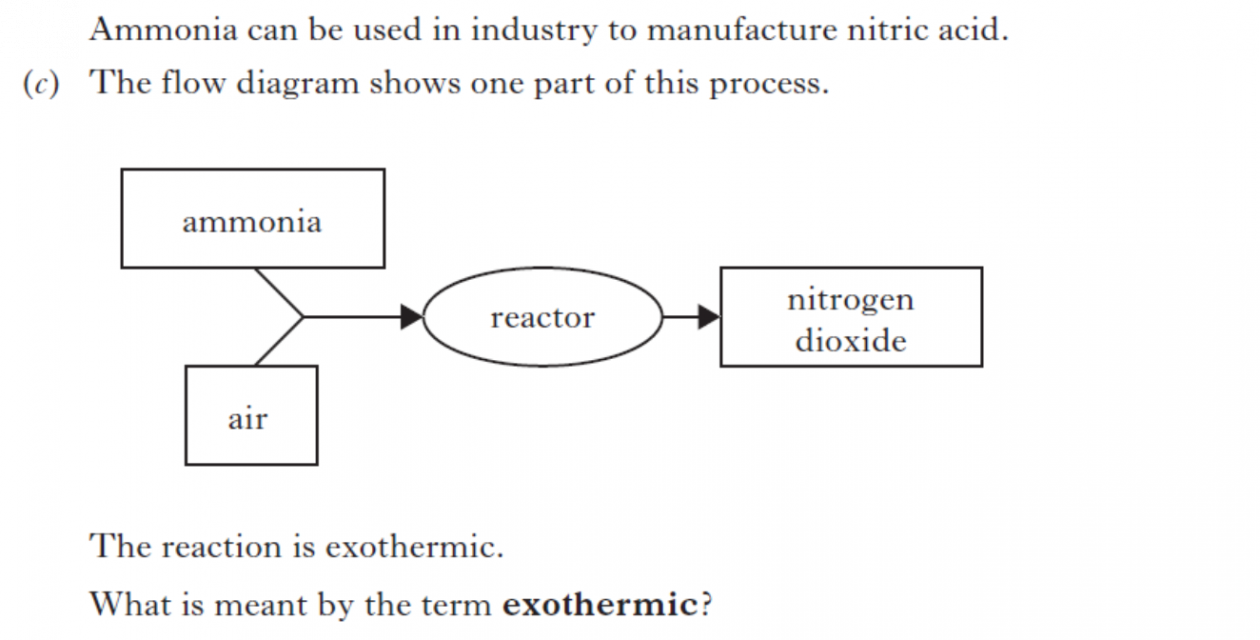 |
|||
| GEN 2013/167 | |||
NOW CHECK YOUR ANSWERS |
 |

