| |
Question 1. |
|
Matthew wanted to find out which type of marble would produce 20ml of gas the fastest when added to sulphuric acid |
|
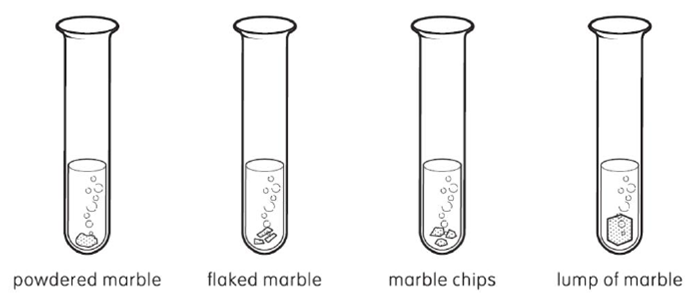 |
|
The time taken to produce the quantity of gas is shown in the table below. |
|
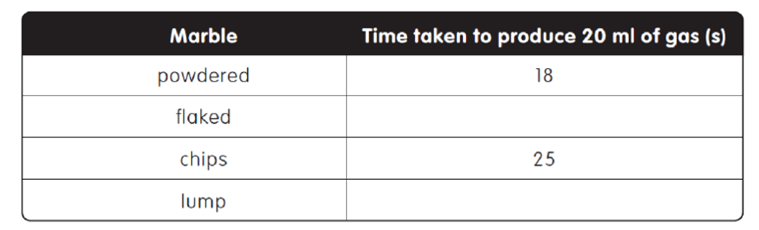 |
| a) |
Which type of marble would be the slowest to produce the 20ml of gas? |
| b) |
Suggest the time that the flaked and lump chips would take to produce 20ml of gas |
| c) |
If 4g of powdered marble was used instead of 2g, how long would the gas have taken to produce? |
|
|
|
Question 2. |
|
What happens in all chemical reactions? |
|
Question 3. |
|
An enquiring scientist found three gas jars with sealed lids. He remembered they contained three different gases, but the labels had fallen off. |
|
 |
|
The gas in jar A relit a glowing splint.
The gas in jar B put out a burning taper with a pop.
The gas in jar C turned limewater milky. |
|
Identify the three gases, A, B and C. |
|
Question 4. |
|
A student set up four experiments to investigate the reaction between zinc and dilute hydrochloric acid. Which two experiments show how changing the particle size affects the speed of the reaction? |
|
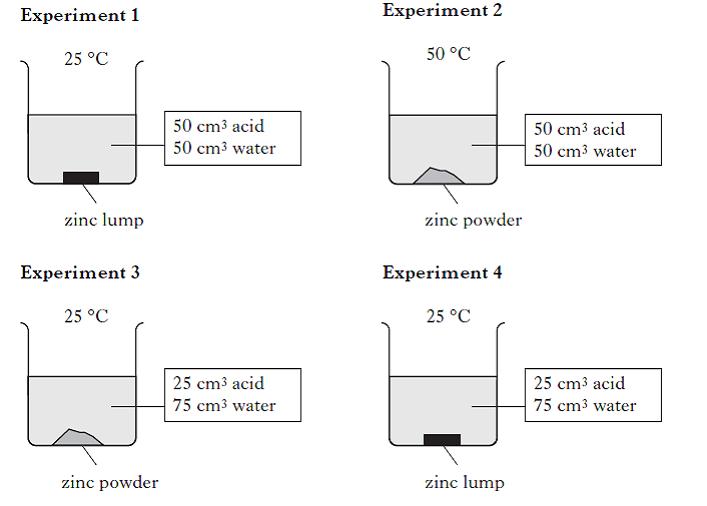 |
| A |
1 and 2 |
| B |
2 and 3 |
| C |
3 and 4 |
| D |
4 and 1 |
|
Question 5. |
|
What element is represented by the symbol Hg. Use the periodic table you have in your homework diary. (if you don’t have your diary find a periodic table on the internet) |
| A |
mercury |
| B |
silver |
| C |
hydrogen |
| D |
helium |

