| |
Question 1. |
|
 |
|
Question 2. |
|
In a class, a student accidentally poured sulphur into a jar of iron filings. She decided it would be easiest to separate the two using a magnet. This method would work because |
| A |
The elements have formed a compound and so are not joined to each other |
| B |
The elements have formed a mixture and so are joined to each other |
| C |
The elements have formed a mixture and so are not joined to each other |
| D |
The elements have formed a compound and are joined to each other |
|
Question 3. |
|
Look at the two different particles on. |
| a) |
In your jotter, draw what you think a compound between these two particle would look like. |
| b) |
The particles make a compound called magnesium oxide. What two elements would be used in making this compound? |
| c) |
From what are compounds made? |
|
Question 4. |
|
Because zinc can combine with other substances but cannot be changed into a simpler substance by an ordinary chemical process, zinc is classified as — |
| A |
a compound |
| B |
an acid |
| C |
an element |
| D |
a mixture |
|
Question 5. |
|
The structure of substances can be shown as models. Which of the models below show an element? |
|
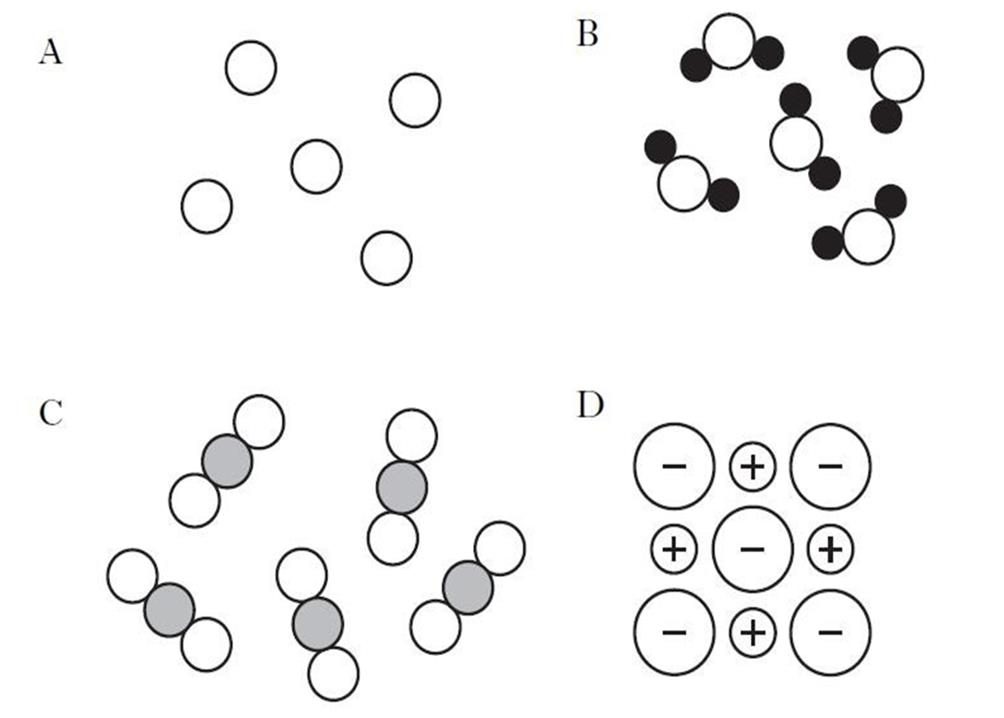 |
|
Question 6. |
|
In a table, divide the list of substances below into 2 groups under the headings:
elements and compounds. |
|
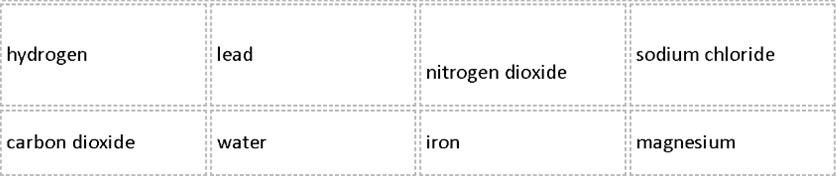 |






