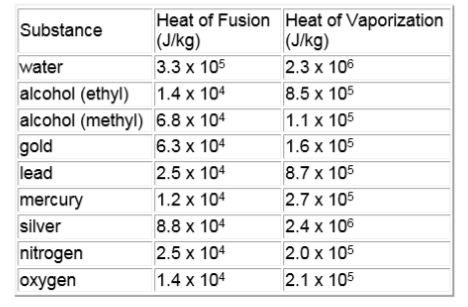
1. Which takes more heat, melting ice or evaporating water?
2. Explain why ice is useful in coolers.
3. How much heat is given off when 3.2 kg of water freeze? Assume the temperature stays constant at 0ºC.
4. How much heat is required to change 2.5 kg of water at 20.0 ºC to steam at 150 ºC?
5. How much heat is needed to change 2.0 kg of silver at 20. ºC into liquid silver? Silver melts at 961ºC.
6. How much heat is needed to change .50kg of ice at –20.ºC into steam at 120ºC?
7. How much heat is released when 500.g of steam at 250 ºC is cooled to ice at –40. ºC?
8. How much heat must be added to a 25g ice cube at 0ºC to change it to water at 0ºC?
9. How much heat is lost when 0.10kg of steam at 100.ºC condenses to water at 80. ºC?
10. How much heat is needed to change 0.10kg of ice at –20.ºC. to steam at 110ºC?