 1. What is the difference between heat and temperature?
1. What is the difference between heat and temperature?
2. What is meant by the following statement:
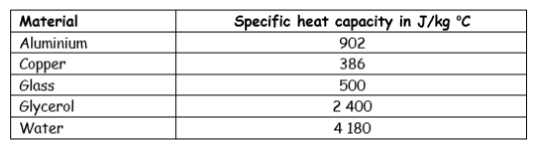
“The specific heat capacity of water is 4180 J / kg °C.”
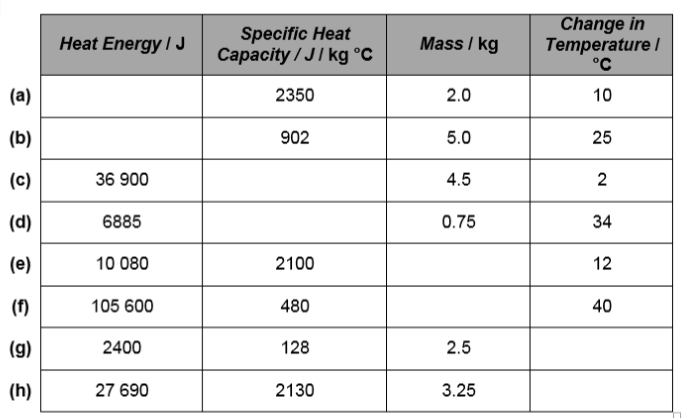
3. Copy and complete this table:
4. What is the heat energy required to heat 3.0 kg of water from 20 °C to 80°C?
5. A 2.4 kg lump of brass is heated up by a Bunsen burner. When 9120 J of heat energy has been absorbed, the temperature of the brass increases by 10 °C. What is the specific heat capacity of the brass?
 6. A pane of glass has a mass of 800 g. What is the temperature change of the glass if it is heated by 1000 J of heat energy?
6. A pane of glass has a mass of 800 g. What is the temperature change of the glass if it is heated by 1000 J of heat energy?
7. A block of lead is heated from 24 °C to 28°C by a heat source that gives off 6144 J of heat energy. What is the mass of the lead block?
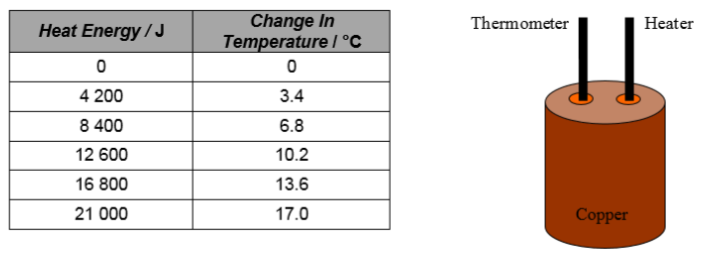
8. In an experiment, a 2 kg block of copper is warmed with a 70 W electrical immersion heater. The temperature of the copper is measured every minute using a thermometer. The heat energy used is calculated by finding the power of the heater and using E = P t. The results are shown.
(a) Using this data, draw a line graph and use the gradient of the straight line to find the specific heat capacity of copper.
(b) Is this experimental value for the specific heat capacity of copper larger, smaller or the same as the actual value? Explain any difference.