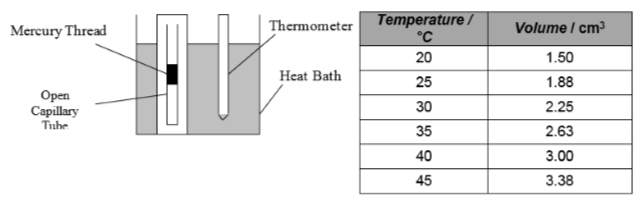
1. In an experiment, an open capillary tube with a mercury thread is placed in to a heat bath.
As the temperature of the gas increases, the mercury thread moves up the capillary tube. The pressure of the gas remains constant because the capillary tube is open.
The temperature of the gas is measured with a thermometer and the volume of the gas is measured using a scale next to the open capillary tube. The results of the experiment are shown.
(a) Draw a rough graph of volume against temperature (in degrees Celsius).
(b) Draw a rough graph of volume against temperature (in Kelvin).
(c) What do these two graphs tell you about the relationship between the volume and temperature of a fixed mass of gas?
(d) Explain this relationship in terms of the kinetic theory of particles.
2. The volume of a fixed mass of gas is 30.0 cm3; at 30 °C. The temperature of the gas is increased to 60 °C without changing the pressure.
A student makes this statement:
‘As the temperature of the gas has doubled, the volume of the gas will also double. Therefore, the volume of the gas at 60 °C will be 60.0 cm3;.’
(a) Explain why this statement is incorrect.
(b) Calculate what the volume of the gas would actually be at 60 °C.
3 .Air is trapped in a glass capillary tube by a bead of mercury. The volume of air is found to be 0.15 cm3 at a temperature of 27 °C.
Assuming that the pressure of the air remains constant, what is the volume of the air at a temperature of 87 °C?
 4 .A fixed mass of gas is trapped in to a syringe. The gas has a pressure of 1.63 x 105 Pa when it has a volume of 3.0 cm3 and a temperature of 22 °C.
4 .A fixed mass of gas is trapped in to a syringe. The gas has a pressure of 1.63 x 105 Pa when it has a volume of 3.0 cm3 and a temperature of 22 °C.
The gas is then heated until it has a uniform temperature of 57 °C. What will be the pressure of the gas if the volume of the gas is increased to 5.0 cm3?