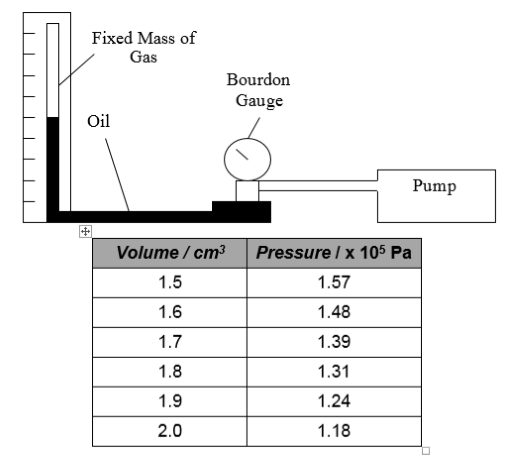
1. In an experiment, the volume of a fixed mass of gas is decreased by trapping the gas at the top of a glass tube with a quantity of oil and then using a pump to push the oil up the tube.
The pressure of the gas is measured with a Bourdon gauge and the volume of gas is measured using a calibrated scale next to the glass tube. The results are shown.
(a) Draw a rough graph (no values needed) of volume against 1 / pressure.
(b) What does this graph tell you about the relationship between the volume and pressure of a fixed mass of gas?
(c) Explain this relationship in terms of the kinetic theory of particles.
2. Explain, using the appropriate gas law, why a balloon will burst if you squeeze it.
3. A 5 cm3 syringe is filled with air and the pressure of the air is found to be 1.01 x 105 Pa. The syringe plunger is then pushed until there is 3 cm3 of air. What is the new air pressure?
4. A scuba diving air tank has a volume of 7.5 litres and is filled with air at a pressure of 1.21 x 107 Pa.  What volume of air will be released by the tank at atmospheric pressure (1.01 x 105 Pa)?
What volume of air will be released by the tank at atmospheric pressure (1.01 x 105 Pa)?