1. Explain, using the kinetic theory of particles, what happens to the particles in a liquid when it melts and becomes a gas.
 2. Explain, using kinetic theory, how the air in a bicycle tyre creates pressure on the inside surface of the tyre.
2. Explain, using kinetic theory, how the air in a bicycle tyre creates pressure on the inside surface of the tyre.
3. Why does the Kelvin temperature scale start at -273 °C?
4. Convert these temperatures from degrees Celsius to Kelvin.
![]() (a) 0 °C
(a) 0 °C
(b) 20 °C
(c) -273 °C
(d) 100 °C
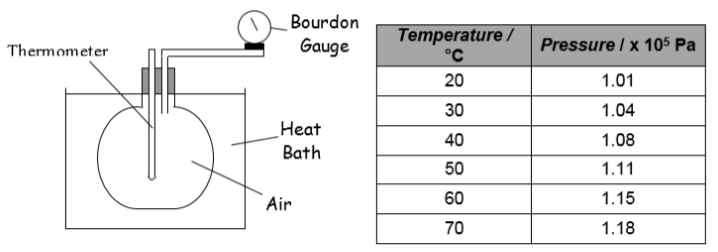
5. A conical flask is sealed with air inside, and is placed in a heat bath. The temperature of the gas increases from 20 °C to 70°C. After every 10 °C temperature increase, the pressure of the gas is measured using a Bourdon gauge. The results are shown.
(a) Draw a rough graph (no values required just the shape) of pressure against temperature (in degrees Celsius).
(b) Draw a rough graph (no values required just the shape) of pressure against temperature (in Kelvin).
(c) What do these two graphs tell you about the relationship between the pressure and temperature of a fixed mass of gas?
(d) Explain this relationship in terms of the kinetic theory of particles.
6. Explain, using the appropriate gas law, why it is important that car tyres are not filled up with so much air that the air pressure is above the car manufacturer’s guidelines?
7. At a temperature of 20 °C, the pressure of a fixed mass of gas in a sealed container is found to be 104 kPa. The gas is heated to a uniform temperature of 90 °C using a heat bath.
What is the pressure of the gas at a temperature of 90 °C?
 8. The pressure of the air in a lorry tyre is found to be 2.58 x 105 Pa at the end of a journey.
8. The pressure of the air in a lorry tyre is found to be 2.58 x 105 Pa at the end of a journey.
Once the tyre has cooled down, the temperature of the air inside the tyre is found to be 10 °C with the pressure decreasing to 2.41 x 105 Pa.
What was the temperature of the air in the tyre at the end of the journey? Give your answer in degrees Celsius.