1. Copy and complete the table, showing the structure of the atom.

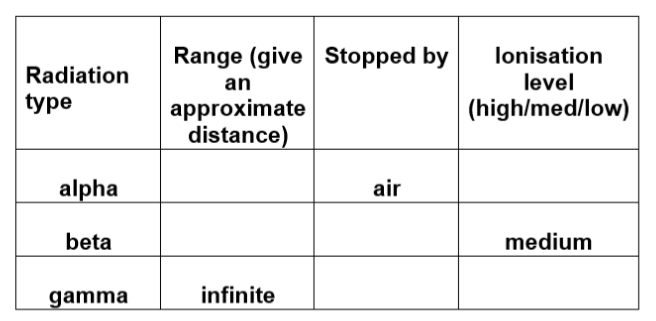
2. Copy and complete the following table, showing the properties of the nuclear radiations.
3. When radiation passes through a material it can ionise the atoms of that material. Explain what is meant by the ionisation of atoms.
4. In the nuclear industry, workers are required to wear film badges to monitor their radiation levels. Explain in detail how film badges are used to monitor exposure to radiation levels.
5. What is the unit used to measure the activity of a radioactive source?
6. Physicists can measure various quantities in nuclear medicine.
Suggest a reason why the energy given out by a source might not be the most helpful quantity to measure for a doctor.
7. Medical physicists and health and safety officers measure “absorbed dose”, D.
Does this quantity relate to the source or the patient and what exactly does it measure?
8. As you know, one hertz could be described as one vibration per second.
How could you describe a gray?
9. Why did someone invent “equivalent dose”, H; wasn’t “absorbed dose”, D good enough for everything? State and explain the difference between ‘equivalent dose’ and ‘absorbed dose’.
10. Traffic ‘activity’ could be described as the ‘number of vehicles passing in an hour’. What is the activity in nuclear medicine and does this refer to the source or the patient?
11. As you should know by now, one hertz could be described as one vibration per second. Describe a becquerel in a similar way.
12. State three factors that the biological effect of radiation depends upon.
13. What is the unit that is used to measure equivalent dose?
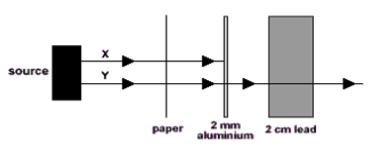
14. Two radioactive sources are fired at different absorbing materials as shown below. Name the types of radiation X & Y.
.