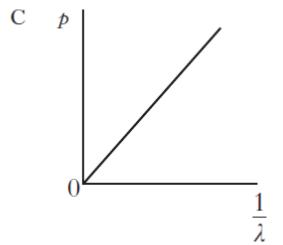| 1. | Three students each make a statement about antiparticles. I An antiparticle has the same mass as its equivalent particle. II An antiparticle has the same charge as its equivalent particle. III Every elementary particle has a corresponding antiparticle. Which of the statements is/are correct? |
||
| A. | I only | ||
| B. | II only | ||
| C. | I and III only | ||
| D. | II and III only | ||
| E. | I, II and III | ||
| 3. |
Part of a radioactive decay series is shown in the diagram. The symbols X1 to X5 represent nuclides in this series.
|
||
| A. | I only | ||
| B. | II only | ||
| C. | I and III only | ||
| D. | II and III only | ||
| E. | I, II and III | ||
Flag this question for later review |
|||
| 4. | A student writes the following statements about electric fields. I There is a force on a charge in an electric field. II When an electric field is applied to a conductor, the free electric charges in the conductor move. III Work is done when a charge is moved in an electric field. Which of the statements is/are correct? |
|
| A. | I only | |
| B. | II only | |
| C. | I and III only | |
| D. | II and III only | |
| E. | I, II and III | |
Flag this question for later review |
||
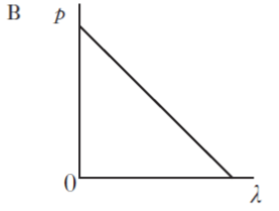
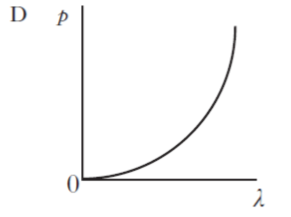
| 5. | All particles exhibit wave properties. The momentum p of a particle is inversely proportional to its wavelength λ. Which of the following graphs shows the relationship between p and λ? |
||
| A. |
|
||
| B. |
|
||
| C. |
|
||
| D. |
|
||
| E. |
|
||