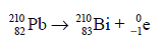2.3 Nuclear Reactions
To examine nuclear reactions it is necessary to define a number of terms used to describe a nucleus.
Nucleon
A nucleon is a particle in a nucleus, i.e. either a proton or a neutron.
Atomic Number
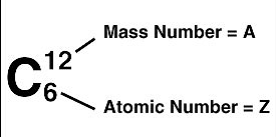
The atomic number, Z, equals the number of protons in the nucleus. In a chemical symbol for an element it is written as a subscript before the element symbol
Example: There are 92 protons in the nucleus of a uranium atom so we write 92U
Mass Number
The mass number, A, is the number of nucleons in a nucleus. In a chemical symbol for an element it is written as a superscript before the element symbol.
Example: One type of atom of uranium has 235 nucleons so we write 235U
Each element in the periodic table has a different atomic number and is identified by that number. It is possible to have different versions of the same element, called isotopes. An isotope of an atom has the same number of protons but a different number of neutrons, i.e. the same atomic number but a different mass number. An isotope is identified by specifying its chemical symbol along with its atomic and mass numbers. For example:
Radioactive Decay
Radioactive decay is the breakdown of a nucleus to release energy and matter from the nucleus. This is the basis of the word ‘nuclear’. The release of energy and/or matter allows unstable nuclei to achieve stability. Unstable nuclei are called radioisotopes or radionuclides.
Representation of decay by symbols and equations
In the following equations, both mass number and atomic number are conserved, i.e. the totals are the same before and after the decay. The original radionuclide is called the parent and the new radionuclide produced after decay is called the daughter product.
Alpha decay
Uranium 238 decays by alpha emission to give Thorium 234
Mass number decreases by 4, atomic number decreases by 2 (due to loss of 2 protons and 2 neutrons).
Alpha decay usually occurs in heavy nuclei such as uranium or plutonium, and therefore is a major part of the radioactive fallout from a nuclear explosion. Since an alpha particle is relatively more massive than other forms of radioactive decay, it can be stopped by a sheet of paper and cannot penetrate human skin. A 4 MeV alpha particle can only travel a few centimetres through the air.
Although the range of an alpha particle is short, if an alpha decaying element is ingested, the alpha particle can do considerable damage to the surrounding tissue. This is why plutonium, with a long half-life, is extremely hazardous if ingested.
Beta decay
Atoms emit beta particles through a process known as beta decay. Beta decay occurs when an atom has either too many protons or too many neutrons in its nucleus. Two types of beta decay can occur. One type (positive beta decay) releases a positively charged beta particle, called a positron, and a neutrino; the other type (negative beta decay) releases a negatively charged beta particle, called an electron, and an antineutrino. The neutrino and the antineutrino are high-energy elementary particles with little or no mass and are released in order to conserve energy during the decay process. Negative beta decay is far more common than positive beta decay.
Lead 210 decays by beta emission to give Bismuth 210.
Mass number is unchanged, atomic number increases by 1.
Gamma decay
Gamma rays are a type of electromagnetic radiation that results from a redistribution of electric charge within a nucleus. Gamma rays are essentially very energetic X – rays; the distinction between the two is not based on their intrinsic nature but rather on their origins. X rays are emitted during atomic processes involving energetic electrons. Gamma radiation is emitted by excited nuclei or other processes involving subatomic particles; it often accompanies alpha or beta radiation, as a nucleus emitting those particles may be left in an excited (higher-energy) state.
Gamma rays are more penetrating than either alpha or beta radiation, but less ionising. Gamma rays from nuclear fallout would probably cause the largest number of casualties in the event of the use of nuclear weapons in a nuclear war. They produce damage similar to that caused by X-rays, such as burns, cancer and genetic mutations.
Example
Thorium 230 decays into Radon. State the name of the particle emitted and give the equation for this decay. (Atomic number of Thorium is 90 and that of Radon is 88).
Solution
Because the atomic number of Radon is less than Thorium, an alpha particle must have been emitted.
Nuclear Fission
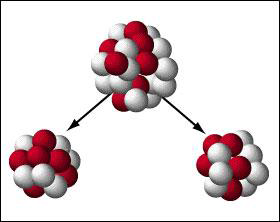
Fission occurs when a heavy nucleus disintegrates, forming two nuclei of smaller mass number. This radioactive decay is spontaneous fission. In this decay process, the nucleus will split into two nearly equal fragments and several free neutrons. A large amount of energy is also released. Most elements do not decay in this manner unless their mass number is greater than 230.
The stray neutrons released by a spontaneous fission can prematurely initiate a chain reaction. This means that the assembly time to reach a critical mass has to be less than the rate of spontaneous fission. Scientists have to consider the spontaneous fission rate of each material when designing nuclear weapons or for nuclear power. For example, the spontaneous fission rate of plutonium 239 is about 300 times larger than that of uranium 235.
Fission can also be induced (persuaded to happen) by neutron bombardment.
This is what happens in a nuclear reactor and is given by the equation;
Mass number and atomic number are both conserved during this reaction. Even though the mass number is conserved, when the masses before and after the fission are compared accurately, there is a mass difference. The total mass before fission is greater than the total mass of the products.
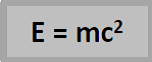
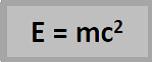
Einstein suggested that mass was a form of energy, and that when there was a decrease in mass, an equivalent amount of energy was produced.
Energy In A Fission Reaction
Einstein’s famous equation shows how mass and energy are related;
In fission reactions, the energy released is carried away as kinetic energy of the fission products.
Fission reactions take place in nuclear reactors. The neutrons released are fast moving. A moderator, e.g. graphite, is used to slow them down and increase the chance of further fissions occurring.
A chain reaction refers to a process in which neutrons released in fission produce an additional fission in at least one further nucleus. This nucleus in turn produces neutrons, and the process repeats. The process may be controlled (nuclear power) or uncontrolled (nuclear weapons).
These slow (thermal) neutrons cause a chain reaction so that more fissions occur. Control rods, e.g. boron, absorb some of the slow neutrons and keep the chain reaction under control. The kinetic energy of the fission products converts to heat in the reactor core.
Example
Calculate the energy released during this fission reaction
Nuclear fission in nuclear reactors
Controlled fission reactions take place in nuclear reactors. The neutrons released are fast moving.
A moderator, eg graphite is used to slow them down and increase the chance of further fissions ccurring.
These slow (thermal) neutrons cause a chain reaction so that more fissions occur.
Control rods, eg boron, absorb some of the slow neutrons and keep the chain reaction under control.
The energy of the moving fission products is transferred by heating in the reactor core.
A coolant fluid (liquid or gas) is required to avoid the core overheating and in addition it can act as a
moderator.
The fluid turns into steam and this drives the turbines.
Fission reactors require containment within reinforced concrete and lead-lined containers to reduce contamination.
Nuclear Fusion
Nuclear energy can also be released by the fusion of two light elements (elements with low atomic numbers).
In a hydrogen bomb, two isotopes of hydrogen, deuterium and tritium are fused to form a nucleus of helium and a neutron.
Unlike nuclear fission, there is no limit on the amount of the fusion that can occur. The immense energy produced by our Sun is as a result of nuclear fusion.
Very high temperatures in the Sun (2.3 × 107 K according to NASA) supply sufficient energy for nuclei to overcome repulsive forces and fuse together. When nuclei fuse, the final mass is less than the initial mass, ie there is a mass difference or mass defect. The energy produced can be calculated using;
Fusion Example
The nuclear reaction can be represented by:
Once again it is found that the total mass after the reaction is less than the total mass before. This reduction in mass appears as an increase in the kinetic energy of the particles.
A Fusion Reactor
Fusion has been successfully achieved with the hydrogen bomb. However, this was an uncontrolled fusion reaction and the key to using fusion as an energy source is control.
The Joint European Torus (JET), in Oxfordshire, is Europe’s largest fusion device. In this device, deuterium–tritium fusion reactions occur at over 100 million kelvin. Even higher temperatures are required for deuterium–deuterium and deuterium–helium 3 reactions
To sustain fusion, 3 conditions must be met at the same time.
- Extremely high plasma temperature (T): 100–200 million K
- A stable reaction lasting at least 5 seconds. This is called the energy confinement time (t)
- A precise plasma density of around 1020 particles/m3
(This is one thousandth of a gram/m3 = one millionth the density of air).
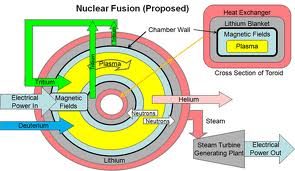
One type of fusion reactor is called a Tokomak. In this design the plasma is heated in a torus or “doughnut-shaped” vessel.
The hot plasma is kept away from the vessel walls by applied magnetic fields. This is shown in the diagram on the right.
One of the main requirements for fusion is to heat the plasma particles to very high temperatures or energies. The methods on the following page are typically used to heat the plasma – all of them are employed on JET.
Induced current
The main plasma current is induced in the plasma by the action of a large transformer. A changing current in the primary coil induces a powerful current (up to 5 million amperes on JET) in the plasma, which acts as the transformer secondary circuit.
Neutral beam heating
Beams of high energy, (neutral) deuterium or tritium atoms, are injected into the plasma, transferring their energy to the plasma via collisions with the ions.
Radio-frequency heating
Electromagnetic waves of a frequency matched to the ions or electrons are able to energise the plasma particles. This is similar to the accelerating structures in a particle accelerator.
Self-heating of plasma
The helium ions (or so-called alpha-particles) produced when deuterium and tritium fuse remain within the plasma’s magnetic trap for a time, before they are pumped away through the diverter. The neutrons (being neutral) escape the magnetic field and their capture in a future fusion power plant will be the source of fusion power to produce electricity.
Breakeven and Ignition
When fusion power out just equals the power required to heat and sustain plasma then breakeven is achieved. However, only the fusion energy contained within the helium ions heats the deuterium and tritium fuel ions (by collisions) to keep the fusion reaction going. When this self-heating mechanism is sufficient to maintain the plasma temperature required for fusion the reaction becomes self-sustaining (ie no external plasma heating is required). This condition is referred to as ignition. In magnetic plasma confinement of the D–T fusion reaction, the condition for ignition is approximately six times more demanding (in confinement time or in plasma density) than the condition for breakeven.