 |
 |
The kinetic energy of the particles can be shown on the graph below.
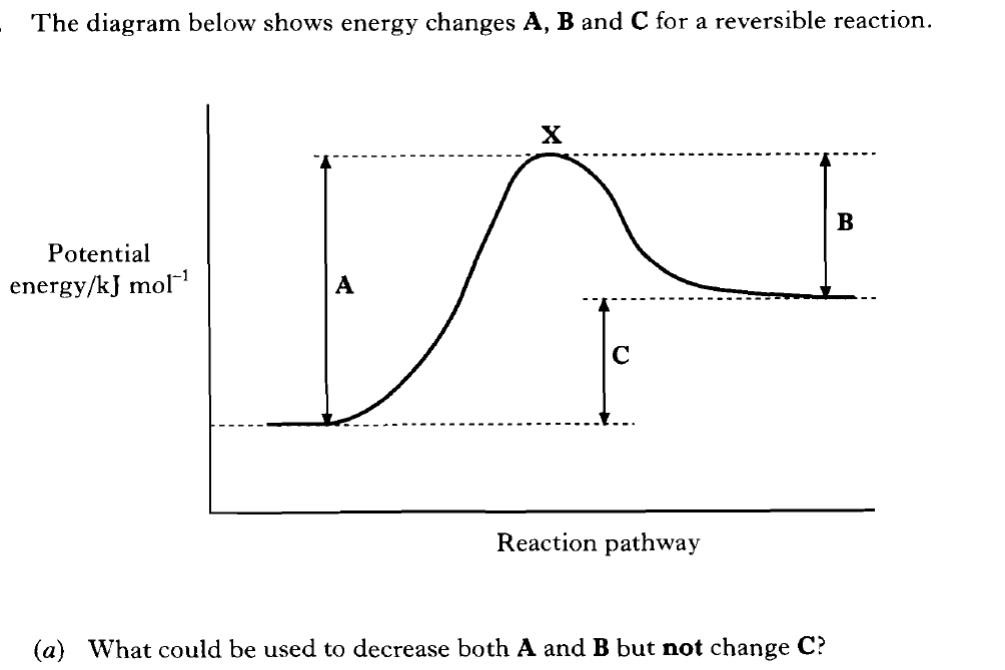
For a given reaction a line can be added showing the activation energy (EA on the graph above). Those particles with kinetic energy greater than the activation energy have the potential to react if a collision occurs. (Not all collisions result in a reaction as the colliding particles must also collide with favourable collision geometry). A catalyst increases the rate of a chemical reaction because it reduces the activation energy (EA) required. Catalysts reduce the height of the peak on the reaction pathway. This will apply to both the forward and back reaction. |


