| 1. | A | |
| 2. | A | |
| 3. | B | |
| 4. | C | |
| 5. | A | |
| 6. | A | |
| 7. | B | |
| 8. | B | |
| 9. | B | |
| 10. | A |
| 11. | |
 |
|
| 12. | |
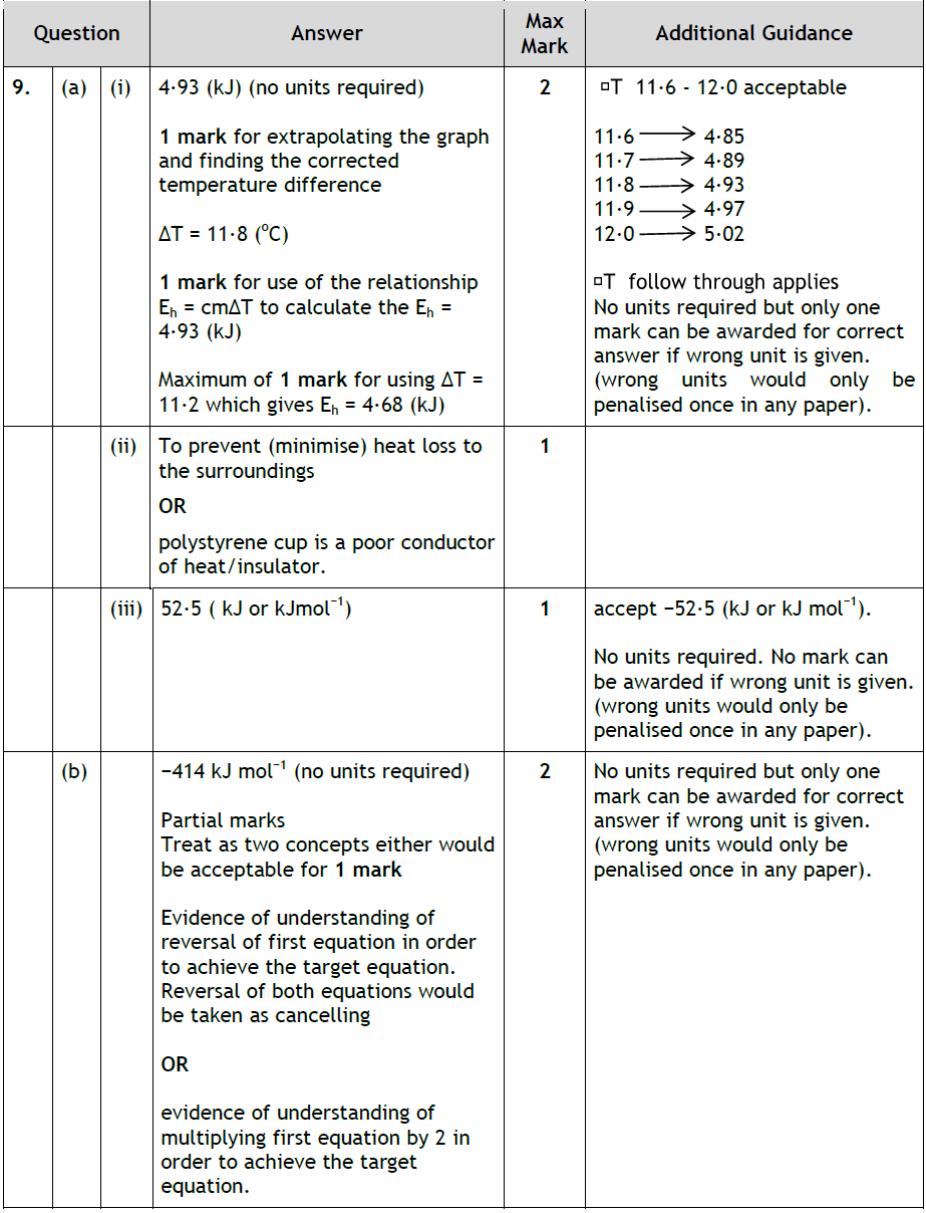 |
|
| 13. | |
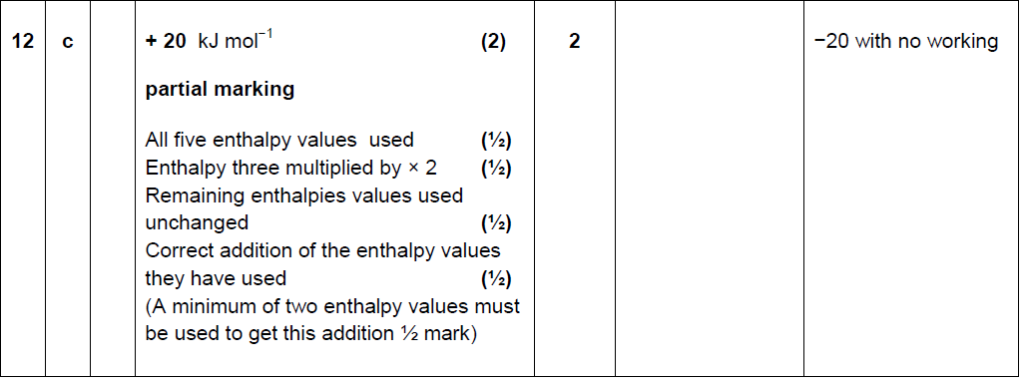 |
|
| 14. | |
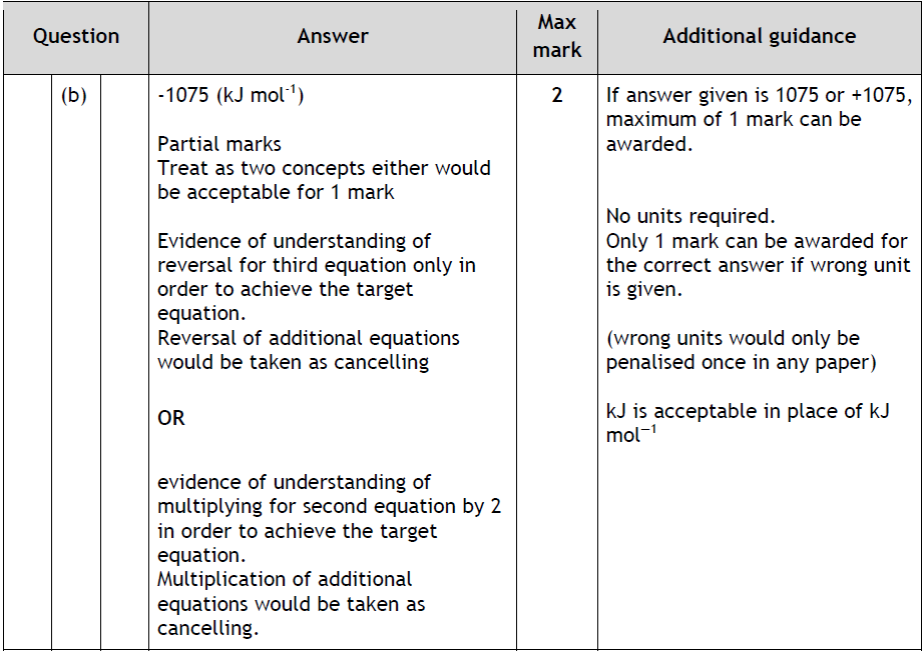 |
|
| 15. | |
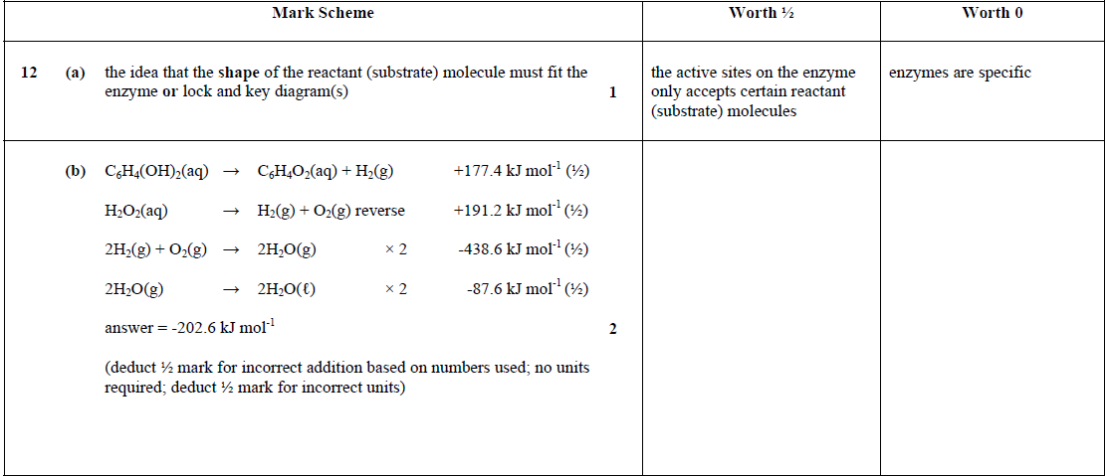 |
|
| 16. | |
 |
|
| 17. | |
| -20 kJ mol-1 | |
| 18. | |
| -236 kJ mol-1 | |
| 19. | |
| -851 kJ mol-1 | |
| 20. | |
| +206 kj mol-1 |
Higher Chemistry Homework Answer Guide
Hyndland Science Faculty
| 1. | A | |
| 2. | A | |
| 3. | B | |
| 4. | C | |
| 5. | A | |
| 6. | A | |
| 7. | B | |
| 8. | B | |
| 9. | B | |
| 10. | A |
| 11. | |
 |
|
| 12. | |
 |
|
| 13. | |
 |
|
| 14. | |
 |
|
| 15. | |
 |
|
| 16. | |
 |
|
| 17. | |
| -20 kJ mol-1 | |
| 18. | |
| -236 kJ mol-1 | |
| 19. | |
| -851 kJ mol-1 | |
| 20. | |
| +206 kj mol-1 |