| 11. |
|
| a) |
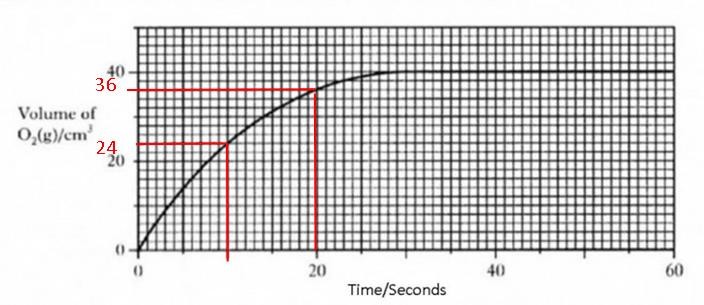 Rate = change / time Rate = change / time
=36-24/10
12/10
1.2 cm3s-1
|
| b) |
 |
|
|
| 12. |
suitable method, tubes must not be blocked |
|
|
| 13. |
|
| a) |
 |
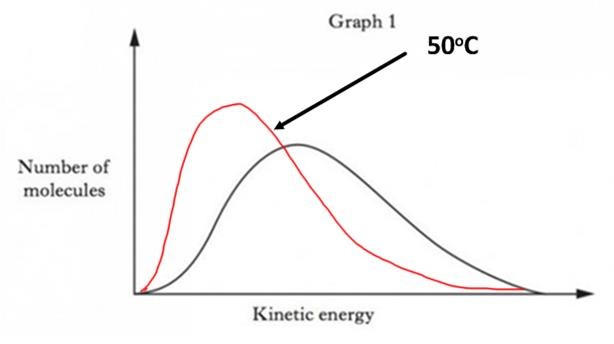
| b) |
 |
|
|
| 14. |
|
| a) |
 |
| b) |
119.8-118.25/5 = 1.55/5 = 0.31 g s-1 |
| c) |
As the reaction proceeds, the reactants are being used up. Consequently, the concentration of reactants decreases. Reducing the concentration of reactants reduces the rate of reaction. |
| d) |
 |
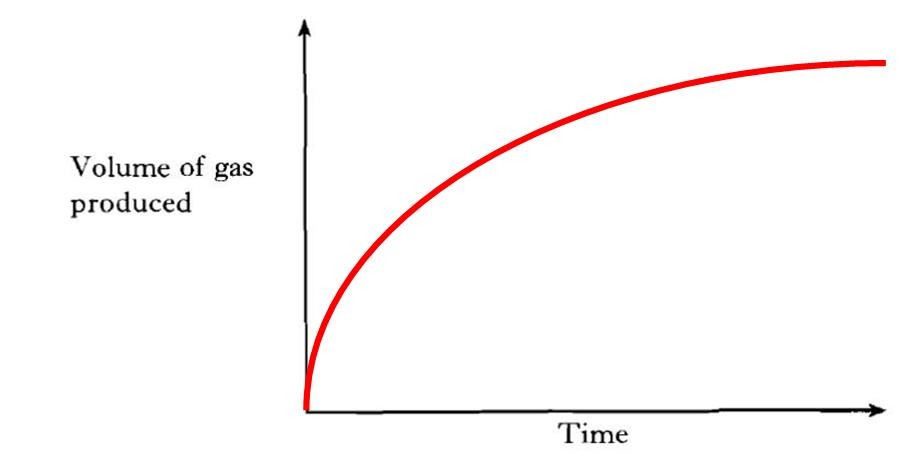
| e) |
 |
| 15. |
|
|
|
|
|
| a) |
Prevents loss of mass due to spitting/ bubbling of liquid out of container |
| b) |
Rate = change/ time
165.00-163.8/10
0.12 g s-1 |
| c) |

|