| 1. 1 pt(s). |
What is the boiling point of water? | |
| A. | 0 C | |
| B. | 37 C | |
| C. | 90 C | |
| D. | 100 C | |
| 2. 1 pt(s). |
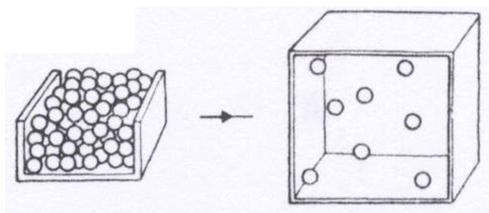
Look at these pictures of molecules. which change is taking place?
|
||
| A. | melting | ||
| B. | freezing | ||
| C. | evaporation (change from a liquid to a gas) | ||
| D. | condensation (change from a gas to liquid) | ||
| 3. 1 pt(s). |
When water changes into a gas, the water molecules | |
| A. | get bigger | |
| B. | get smaller | |
| C. | move faster | |
| D. | move slower | |
| 4. 1 pt(s). |
When water changes into ice, the water molecules | |
| A. | move slower | |
| B. | move faster | |
| C. | get bigger | |
| D. | get smaller | |
| 5. 1 pt(s). |
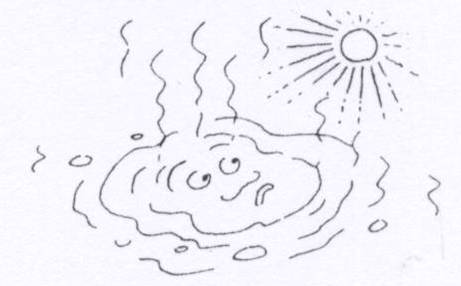
When a puddle dries up, which change is taking place?
|
||
| A. | melting | ||
| B. | freezing | ||
| C. | evaporation (change from a liquid to a gas) | ||
| D. | condensation (change from a gas to liquid) | ||
| 6. 1 pt(s). |
The spaces between molecules in a gas are | |
| A. | bigger than in a solid | |
| B. | smaller than in a liquid | |
| C. | the same size as in a solid | |
| D. | the same size as in a liquid | |
| 7. 1 pt(s). |
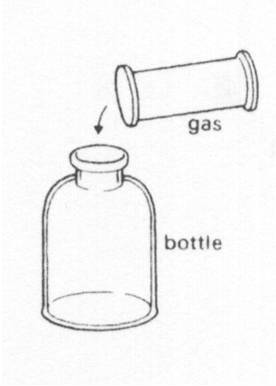
Brown gas is poured from a gas jar into a bottle. After a few minutes, the gas
|
||
| A. | has a smaller volume | ||
| B. | keeps the same shape as the gas jar | ||
| C. | fills the bottle | ||
| D. | keeps the same volume as the gas jar | ||
| 8. 1 pt(s). |
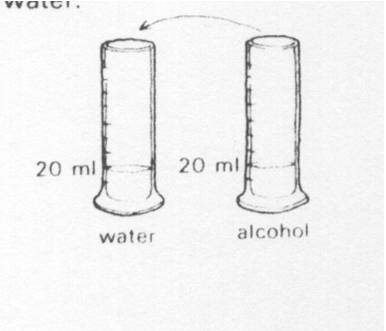
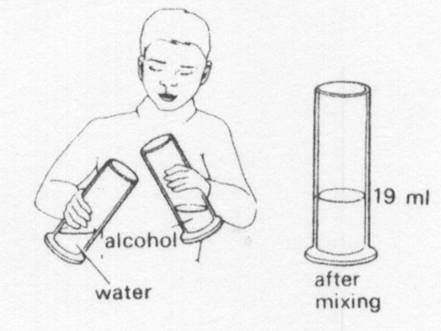
20 ml of alcohol is poured into 20 ml of water. The volume of after mixing could be
|
||
| A. | 20 ml | ||
| B. | 39 ml | ||
| C. | 40 ml | ||
| D. | 45 ml | ||
| 9. 1 pt(s). |
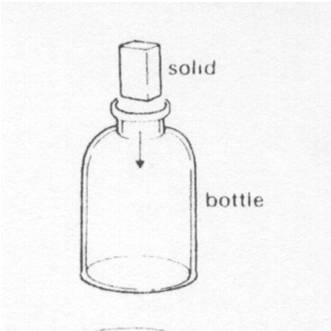
A solid is put into a bottle. The soild now
|
||
| A. | fills the bottle | ||
| B. | has a bigger volume | ||
| C. | has the same volume | ||
| D. | has the shape of the bottle | ||
| 10. 1 pt(s). |
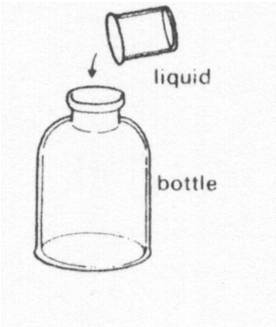
A liquid is poured from a beaker into a bottle. The liquid now
|
||
| A. | fills the bottle | ||
| B. | has a bigger volume | ||
| C. | keeps the same shape | ||
| D. | keeps the same volume | ||
| 11. 1 pt(s). |
10 ml of alcohol is poured into 10 ml of water. After mixing the volume is less than 20 ml. This because the alcohol molecules
|
||
| A. | are all different sizes | ||
| B. | fit between the water molecules | ||
| C. | are identical to the water molecules | ||
| D. | have the saem size as the water molecules | ||
| 12. 1 pt(s). |
Look at the drawings. Which best represents a solid? | ||
| A. |
|
||
| B. |
|
||
| C. |
|
||
| D. |
|
||
| 13. 1 pt(s). |
Look at the drawings. Which best represents a liquid? | ||
| A. |
|
||
| B. |
|
||
| C. |
|
||
| D. |
|
||
| 14. 1 pt(s). |
Look at the drawings. Which best represents a gas? | ||
| A. |
|
||
| B. |
|
||
| C. |
|
||
| D. |
|
||
| 15. 1 pt(s). |
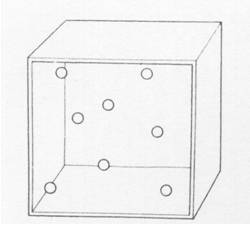
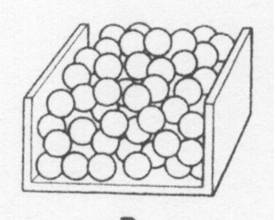
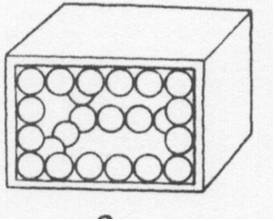
20 ml of sand is poured into 20 ml of peas. The volume after mixing could be
|
||
| A. | 20 ml | ||
| B. | 35 ml | ||
| C. | 40 ml | ||
| D. | 45 ml | ||