| 1. 1 pt(s). |
Which of these metals would be found pure in the earth’s crust | |
| A. | iron | |
| B. | silver | |
| C. | aluminium | |
| D. | magnesium | |
| 2. 1 pt(s). |
According to the graph, which air pollutant decreased the most from 1970 to 1991?
|
||
| A. | Carbon monoxide | ||
| B. | Sulfur oxides | ||
| C. | Particulates | ||
| D. | Lead | ||
| 3. 1 pt(s). |
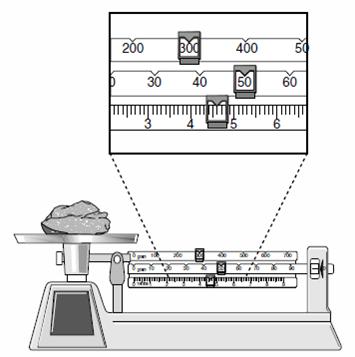
What is the mass of the rock?
|
||
| A. | 335.6 g | ||
| B. | 350.6 g | ||
| C. | 354.6 g | ||
| D. | 356.0 g | ||
| 4. 1 pt(s). |
To obtain a very reactive metal from its compounds requires…………. | |
| A. | little energy | |
| B. | a lot of energy | |
| C. | not much heat | |
| D. | an act of God | |
| 5. 1 pt(s). |
To obtain an unreactive metal from its compounds requires…………. | |
| A. | little energy | |
| B. | a lot of energy | |
| C. | much heat | |
| D. | hope | |
| 6. 1 pt(s). |
Limestone is added to the Blast Furnace to remove impurities. The impurities float on the surface of the molten iron and forms a material called …. | |
| A. | scum | |
| B. | slag | |
| C. | litter | |
| D. | concrete | |
| 7. 1 pt(s). |
We can use the Blast Furnace to extract iron from iron ore but not aluminium from aluminium ore because …… | |
| A. | iron is a less reactive metal and needs less energy to extract it from its ore | |
| B. | iron is a more reactive metal and needs less energy to extract it from its ore | |
| C. | iron is a less reactive metal and needs more energy to extract it from its ore | |
| D. | iron is a more reactive metal and needs more energy to extract it from its ore | |
| 8. 1 pt(s). |
The blast furnace cannot be used to extract aluminium from its ore because | |
| A. | aluiminium is too unreactive a metal and needs the energy of electrolysis to extract it from its ore | |
| B. | aluiminium is too reactive a metal and needs the energy of electrolysis to extract it from its ore | |
| C. | aluiminium is too reactive a metal and only needs gentle heating to extract it from its ore | |
| D. | aluiminium is an unreactive a metal and is found uncombined in the Earth’s crust | |
| 9. 1 pt(s). |
Some metals are found uncombined, in their native form in the ground. These metals would be | |
| A. | hard | |
| B. | soft | |
| C. | unreactive | |
| D. | reactive | |
| 10. 1 pt(s). |
What rocks are you most likely to find beside a volcano? | |
| A. | igneous | |
| B. | metamorphic | |
| C. | sedimentary | |
| 11. 1 pt(s). |
When heat and pressure are applied to a rock, what kind of rock is formed? | |
| A. | sedimentary | |
| B. | igneous | |
| C. | metamorphic | |
| 12. 1 pt(s). |
What is the difference between an ore and a mineral containing a metal element? | |
| A. | an ore canotains only a small amount of the metal, but the mineral has enough to make it economic to extract the metal | |
| B. | a mineral contains only a small amount of the metal, but the ore has enough to make it uneconomic to extract the metal | |
| C. | a mineral contains only a small amount of the metal, but the ore has enough to make it economic to extract the metal | |
| D. | an ore contains only a small amount of the metal, but the mineral has enough to make it uneconomic to extract the metal | |
| 13. 1 pt(s). |
When copper chloride is electrolysed | |
| A. | copper metal forms at the positive electrode and chlorine gas forms at the negative electrode | |
| B. | chlorine gas forms at the positive electrode and chlorine gas forms at the negative electrode | |
| C. | copper metal forms at the positive electrode and copper metal forms at the negative electrode | |
| D. | chlorine gas forms at the positive electrode and copper metal forms at the negative electrode | |
| 14. 1 pt(s). |
When assessing what a soil is made up of, a sample can be shaken and left to settle. The bottom layer that settles out will contain | |
| A. | water | |
| B. | sand | |
| C. | clay | |
| D. | silt | |
| E. | humus | |
| 15. 1 pt(s). |
Four metals, silver, zinc, iron and copper were added to solutions of each of their salts. When iron was added to copper, what would be observed | |
| A. | the iron disappears | |
| B. | the iron disappears and copper appears | |
| C. | the iron bubbles releasing hydrogen gas | |
| D. | no reaction would be seen | |