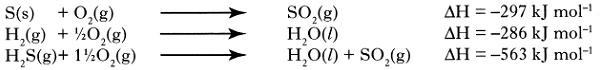- 1. What is the relationship between a, b, c and d? Answer in the form a =
S(s) + H2(g) ® H2S(g) ΔH = a
H2(g) + ½ O2 (g) ® H2O(l) ΔH = b
S(s) + O2(g) ® SO2(g) ΔH = c
H2S(g) + 1 ½ O2(g) ® H2O(l) +SO2(g) ΔH = d
2. The enthalpy changes for the formation of one mole of aluminium oxide and one mole of iron(III) oxide are shown below.
2Al(s) + 1½O2(g) à Al2O3(s) ΔH = -1676 kJ mol-1
2Fe(s) + 1½O2(g) à Fe2O3(s) ΔH = -825 kJ mol-1
Use the above information to calculate the enthalpy change for the reaction:
2Al(s) + Fe2O3(s) à Al2O3(s) + 2Fe(s)
3. The equation for the enthalpy of formation of propanone is:
3C(s) + 3H2(g) + ½O2(g) C3H6O(l)
Use the following information on enthalpies of combustion to calculate the enthalpy of formation of propanone.
C(s) + O2(g) CO2(g) ΔH = -394 kJmol-1
H2(g) + ½O2(g) H2O(l) ΔH = -286 kJmol-1
C3H6O(l) + 4O2(g) 3CO2(g) + 3H2O(l) ΔH = -1804 kJmol-1
4. The equation below represents the hydrogenation of ethene to ethane.
C2H4(g) + H2(g) → C2H6(g)
Use the enthalpies of combustion of ethene, hydrogen and ethane from page 9 of the data booklet to calculate the enthalpy change for the above reaction.
5. Calculate a value for the enthalpy change involved in the formation of one mole of hydrogen peroxide from water (ΔH3). The enthalpy change when hydrogen forms hydrogen peroxide is -188 kJ mol-1 and the enthalpy of combustion of hydrogen to form water is -286 kJ mol-1.
6. Calculate a value for the enthalpy change involved in the decomposition of nitrogen dioxide to nitrogen monoxide given the following information.
Equation (a) N2(g) + O2(g) à 2NO(g) ΔH = +181 kJ
Equation (b) N2(g) + 2O2(g) à 2NO2(g) ΔH = +68 kJ
7. The sulphur-iodine cycle is an industrial process used to manufacture hydrogen. There are three steps in the sulphur-iodine cycle.
Step 1: I2 + SO2 + 2H2O → 2HI + H2SO4
Step 2: 2HI → I2 + H2
Step 3: H2SO4 → SO2 + H2O + ½O2
(i) Why does step 3 help to reduce the cost of manufacturing hydrogen?
(ii) What is the overall equation for the sulphur-iodine cycle?
8. The enthalpy of formation of glycerol is the enthalpy change for the reaction:
3C(s) + 4H2(g) + 1½O2(g) → C3H8O3(ℓ)
(graphite)
Calculate the enthalpy of formation of glycerol, in kJ mol–1, using information from the data booklet and the following data.
C3H8O3(ℓ) + 3½O2(g) → 3CO2(g) + 4H2O(ℓ) ΔH = – 1654 kJ mol–1
Using the data above, calculate the enthalpy change, in kJ mol–1, for the production of butan-2-ol by hydration of but-2-ene.
9. Enthalpy changes can also be calculated using Hess’s Law.
The enthalpy of formation for pentan-1-ol is shown below.
5C(s) + 6H2(g) + O2(g) → C5H11OH( ℓ ) ΔH = –354 kJ mol–1
Using this value, and the enthalpies of combustion of carbon and hydrogen from the data booklet, calculate the enthalpy of combustion of pentan-1-ol, in kJ mol–1.
10. Given that
The enthalphy change for the reaction will be
11. The three equations shown below all involve displacement reactions of metals and metal oxides
What is the relationship between A, B and C according to Hess’s Law
12. Given the equations
then according to Hess’sLaw
13. The equation for the enthapy of formation of ethyne is
Use the values for the enthalphy of combustion of ethyne, carbon and hydrogen given in the data booklet to calculate the enthalphy of formation of ethyne.