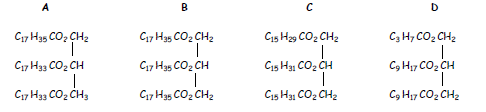1. Fats and oils can be classified as
1. Fats and oils can be classified as
A soaps
B fatty acids
C esters
D polyesters
2. Which of the following decolorises bromine water least successfully?
A palm oil
B hex-1-ene
C cod liver oil
D mutton fat
3. In the formation of “hardened” fats from vegetable oils, the hydrogen
A causes cross-linking between the chains
B causes hydrolysis to occur
C increases the carbon chain length
D reduces the number of carbon to carbon double bonds.
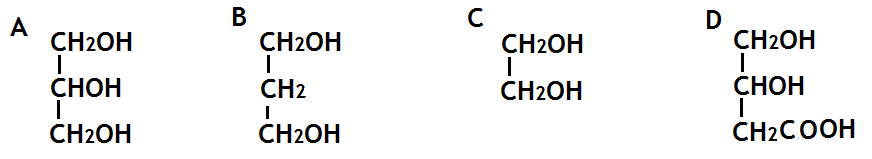
4. The structural formula for glycerol is
5. The production of fatty acids and glycerol from fats in foods is an example of
A hydrolysis
B hydrogenation
C dehydration
D dehydrogenation
6. Explain, in terms of structure, why fats are solids and oils are liquids at room temperature.
7. Foodstuffs have labels that list ingredients and provide nutritional information.
The label on a tub of margarine lists hydrogenated vegetable oils as one of the ingredients.
Why have some of the vegetable oils in this product been hydrogenated?
8. a) Draw the extended structural formula for a molecule of glycerol.
b) What is the systematic name for a molecule of glycerol.
c) Explain why fats are sometimes referred to as triglycerides
d) What do you understand by the term ‘fatty acid’?
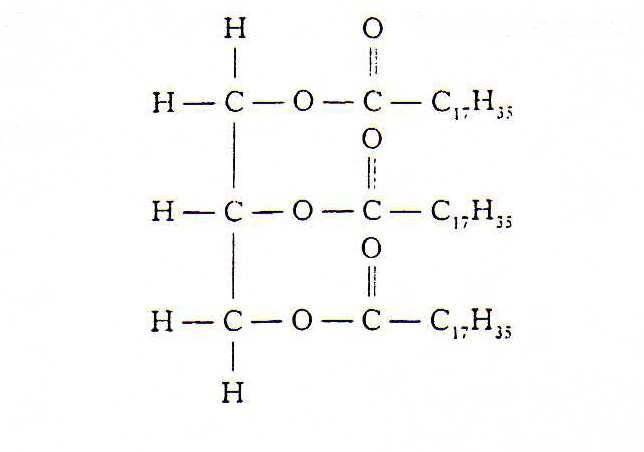
9. The structure of a fat molecule is shown below.
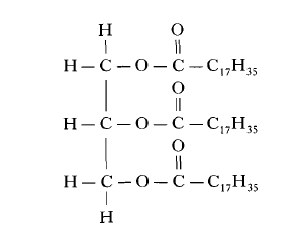
(a) When the fat is hydrolysed, a fatty acid is obtained. Name the other product obtained in this reaction.
(b) Oils are liquid at room temperature; fats are solid. Why do oils have lower melting points than fats?
10. Mutton fat contains a compound called as tristearin.
Tristearin is hydrolysed in the body during digestion by an e nzyme known as lipase.
(a) Give one reason why fats can be a useful part of a balanced diet.
(b) To which set of compounds do enzymes belong?
(c) The hydrolysis of tristearin produces a fatty acid.
Name the other product of the reaction.
11. A triglyceride is best described as
A an ester molecule made by joining three fatty acids and one glycerol molecule
B an ester mixture of three fatty acids and one glycerol molecule
C an ester molecule made by joining three glycerol molecules and one fatty acid molecule
D an ester mixture of three glyceride molcules
12. Both solid and liquid fats are
A mixtures of triglycerides in which the fatty acids are always identical.
B mixtures of different esters.
C mixtures of esters formed from unsaturated acids
D mixtures of triglycerides formed from fatty acids which can be the same or different.
13. On hydrolysis, a fat molecule would produce
A three molecules of glycerol and one molecule of fatty acid.
B three molecules of glycerol and three molecules of fatty acid.
C one molecule of glycerol and three molecules of fatty acids.
D one molecule of glycerol and one molecule of fatty acid.
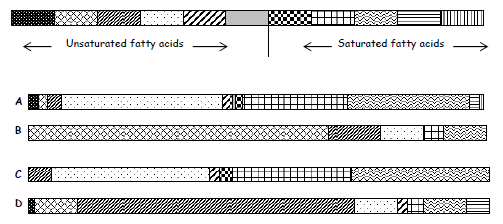
14. The composition of four fats and oils ( A, B, C and D) can be estimated using this key. Each area of shading represents the involvement of a particular fatty acid.
a) Which substance would be the least healthy in terms of diet?
b) Which substance is likely to have the lowest melting point?
c) Which substance is made from the largest number of fatty acids?
15.
a) Which of the above molecules would NOT react rapidly with bromine water?
b) Which of the above molecules would require the least amount of hydrogen for hydrogenation?
c) Place the above molecules in order of increasing melting point.
16. The hydrolysis of a fat produces glycerol and fatty acids.
a) What does the term hydrolysis mean?
b) State the ratio of glycerol molecules to fatty acid molecules.
c) A triglyceride produces only glycerol and palmitic acid, CH3(CH2)14COOH, on hydrolysis.
i) Draw the structural formula for the triglyceride.
ii) Explain whether the triglyceride is likely to be a fat or an oil.
17. Explain why edible oils are sometimes used as lubricants for farm machinery.