| 1. What are the three steps for the reaction between alkanes and halogens? |
|
| 2. Write out each of the three steps for the reaction between chlorine gas and ethane under the action of UV light. |
| 3. What is meant by a free-radical scavenger? |
| 4. Suncreams contain antioxidants. |
a) The antioxidant, compound A, can prevent damage to skin by reacting with free radicals such as NO2•.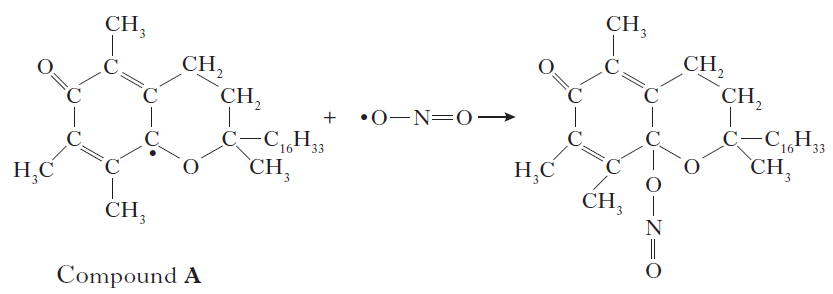 |
| Why can compound A be described as a free radical scavenger in the reaction shown above? |
b) Another antioxidant used in skin care products is vitamin C, C6H8O6.

Copy and complete the ion-electron equation for the oxidation of vitamin C. |
5. Fluorine reacts with methane via a free radical chain reaction.
Some steps in the chain reaction are shown in the table below. |
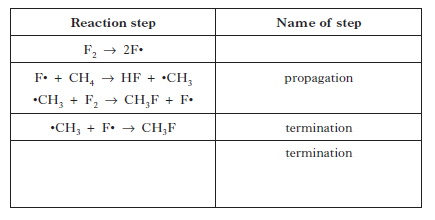 |
Copy and complete the table by:
a) inserting the missing name for the first step;
b) showing another possible termination reaction in the final row of the
table. |