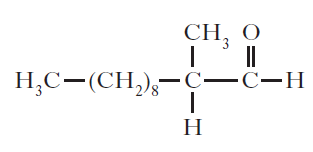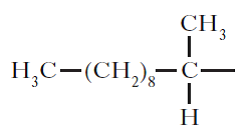![]()
1. Which unit makes up every terpene?
2. How many carbons there are in an isoprene unit?
3. What is the systematic name for isoprene?
4. What is an oxidised terpene known as?
5. Give 3 uses of essential oils.
6. Two typical compounds that are present in many perfumes are shown.
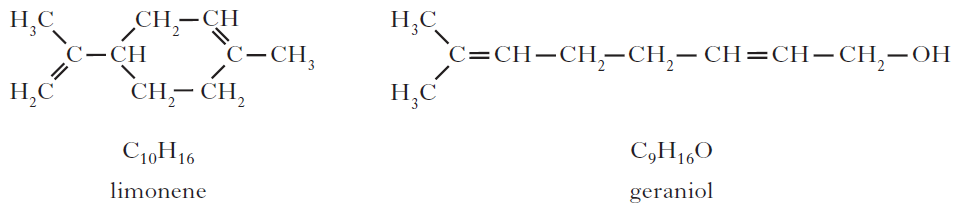
(a) Why does geraniol evaporate more slowly than limonene?
(b) The structure of one of the first synthetic scents used in perfume is shown below.
(i) Name the family of carbonyl compounds to which this synthetic scent belongs.
(ii) Copy and complete the structure below to show the product formed when this scent is oxidised.
7. A team of chemists are developing a fragrance for use in a shower gel for men.
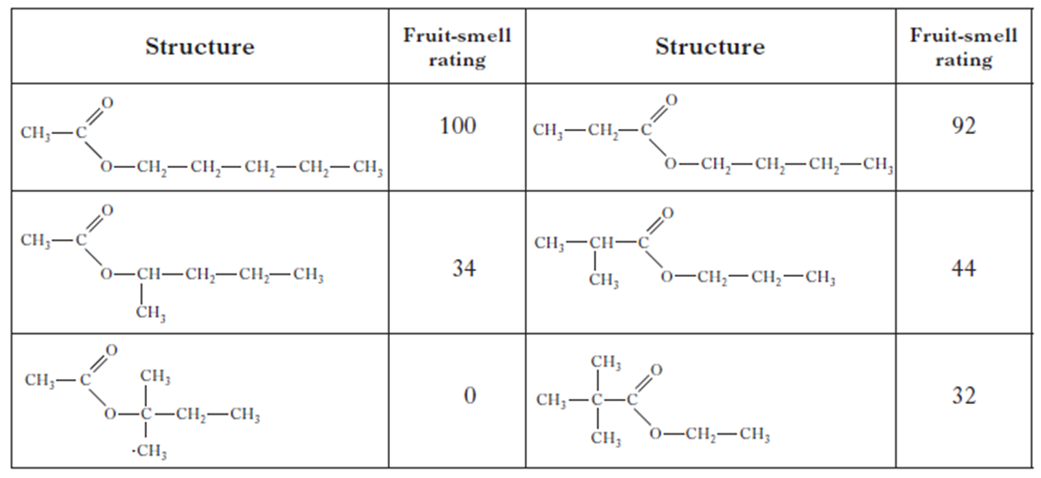
(a) To give the gel a fruity smell the chemists are considering adding an ester.
They synthesise six isomeric esters. Volunteers smell each ester and give it a rating out of one hundred depending on how fruity the smell is.
(i) Name the ester with the fruit-smell rating of 92.
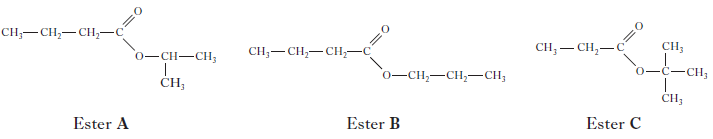
(ii) Shown below are the structures of three more isomers.
Put these esters in order of decreasing fruit-smell rating.
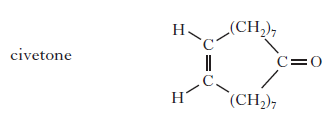
(b) To create a fragrance for men, the compound civetone is added. Draw a structural formula for the alcohol that can be oxidised to form civetone.
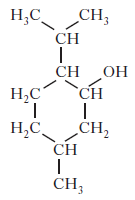
(c) To make the shower gel produce a cold, tingling sensation when applied to the skin, menthol is added. Like terpenes, menthol is formed from isoprene (2-methylbuta-1,3-diene).
Copy the diagram of the structure of menthol below and circle an isoprene unit.
8. Limonene is one of the terpene molecules responsible for the flavour of lemons.
How many isoprene units are used in theproduction of one limonene molecule?
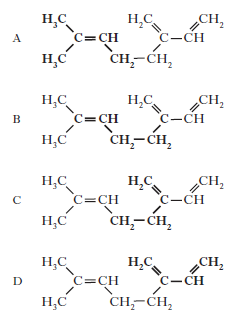
A 1
B 2
C 3
D 4
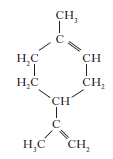
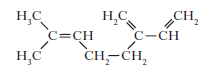
9. Myrcene is a simple terpene.
Terpenes contain at least one isoprene unit.Which of the following shows a correctly highlighted isoprene unit?