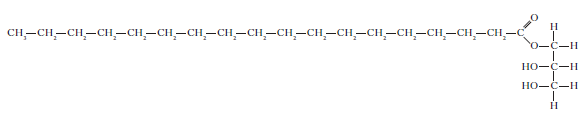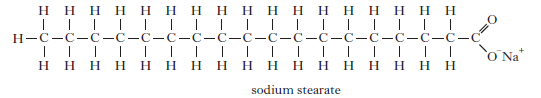![]() 1. Explain what is meant by the terms
1. Explain what is meant by the terms
a) hydrophilic b) hydrophobic.
2. Describe how soap can clean a fat stain from clothing. You should use the following words in your answer:
ionic head, covalent tail, hydrophobic, hydrophilic, polar, non-polar
3. Soap can be produced by the reaction of fats and oils with sodium hydroxide solution.
a) Name the kind of reaction that is taking place.
b) Describe the structure of soap
4. Small children can find it difficult to swallow tablets or pills so ibuprofen is supplied as an “infant formula” emulsion.
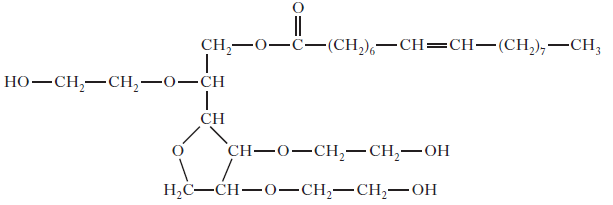
The emulsifier used is polysorbate 80. Its structure is shown below.
Explain why this molecule acts as an emulsifier.
5. Glycerol monostearate is an emulsifier used in chocolate.
a) Why is glycerol monostearate added to chocolate?
b) Draw a structural formula for glycerol.
c) Copy the structure and label the hydrophobic and hydrophilic areas of the molecule
6. Stearic acid reacts with sodium hydroxide solution to form sodium stearate.
(i) Name the type of reaction taking place when stearic acid reacts with
sodium hydroxide.
(ii) Explain fully how sodium stearate acts to keep grease and non-polar
substances suspended in water during cleaning.