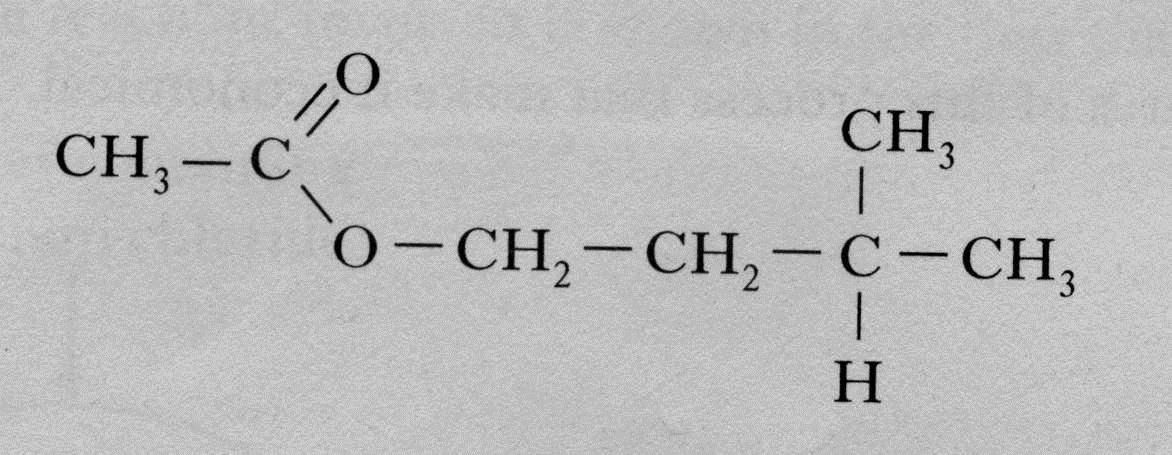![]()
1. Which two types of chemicals react together to produce an ester?
2. Copy the chemical structure shown below and circle the ester link.
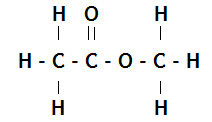
3. Name and draw the structural formula of the ester formed when each of the following chemicals reacts together.
a) ethanol and methanoic acid b) methanol and propanoic acid
c) butanoic acid and pentanol d) ethanoic acid and propanol
4. Which of the following consumer products is least likely to contain esters?
A flavourings
B perfumes
C solvents
D toothpastes
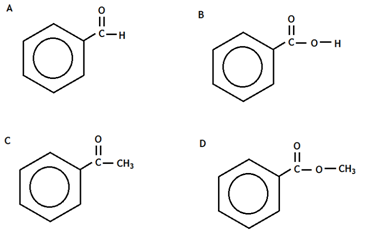
5. Which of these represents the full structural formula of ethyl propanoate?
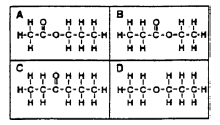
6. Which of these would be formed from propanol and methanoic acid?
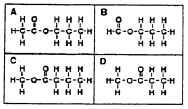
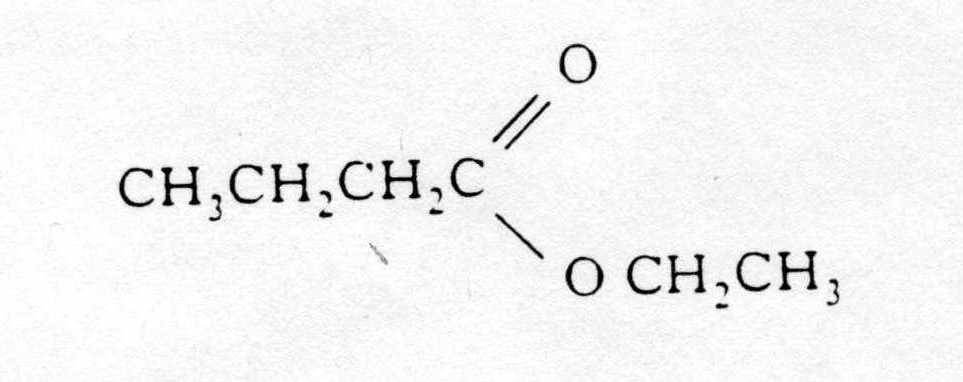
7. Which reactants would react together to produce this molecule?
A Methanoic acid and ethanol
B Ethanoic acid and methanoic acid
C Propanoic acid and water
D Methanol and ethanoic acid
8. Sodium hydrogencarbonate is added to the mixture after esterification to
A remove excess alkanol
B remove excess water
C make the pH more acidic
D remove excess acid
9. In ester preparation which of the following is most commonly used as a
catalyst?
A Concentrated sulphuric acid
B Concentrated hydrochloric acid
C Concentrated nitric acid
D Concentrated phosphoric acid
10. What would hydrolysis of this molecule produce?
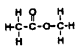
A Propanol and methanoic acid
B Propanoic acid and methanol
C Ethanoic acid and ethanol
D Ethanol and ethanone
11. Draw the structural formula of the esters formed from……………………………
a) methanoic acid and propan-1-ol
b) ethanoic acid and butan-2-ol
c) 
12. Which of the following is an ester?
13. Rum flavouring is based on a compound with the formula shown,
It can be made from
A ethanol and butanoic acid
B propanol and ethanoic acid
C butanol and methanoic acid
D propanol and propanoic acid
14. Give three uses of esters.
15. A pupil made the ester ethyl propanoate in a test tube and poured the reaction mixture into a beaker containing sodium hydrogen-carbonate solution.
a) Name the acid and alcohol used to make the ester.
b) What two things would the pupil observe when the ester is poured into the sodium hydrogen- carbonate solution?
c) The pupil heated the reaction mixture using a hot water bath. Why was the reaction mixture not heated directly with a Bunsen flame?
16. One of the chemicals released in a bee sting is an ester that has the structure shown.
This ester can be produced by the reaction of an alcohol with an alkanoic acid.
(a) Name this acid.
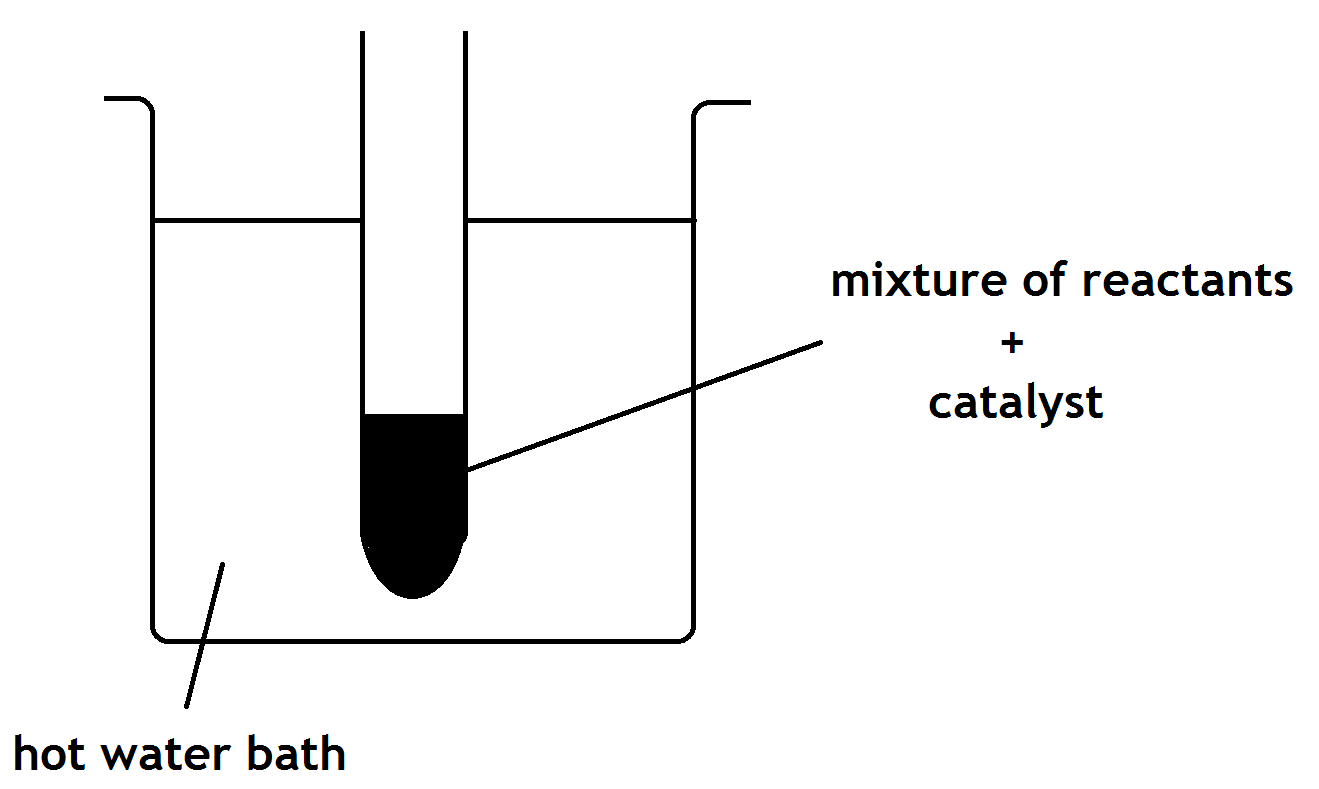 (b) The ester can be prepared in the lab by heating a mixture of the reactants with a catalyst.
(b) The ester can be prepared in the lab by heating a mixture of the reactants with a catalyst.
(i) Name the catalyst used in the reaction.
(ii) What improvement could be made to the experimental setup shown opposite?