  |
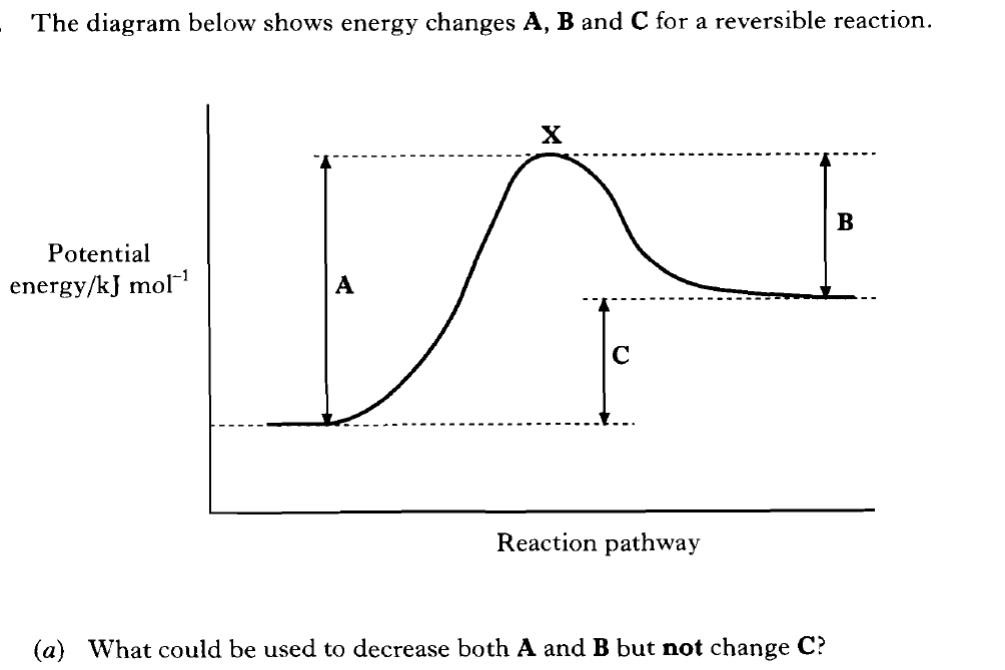
As reactants move to form products, they must go through a transition stage where they are neither reactant nor product as bonds are both being made (to form the products) and broken (to break up reactants). This stage is the highest energy state that the atoms/ molecules will occupy.
This high energy arrangement of atoms/ molecules in a reaction is known as the activated complex. |

