|
 :
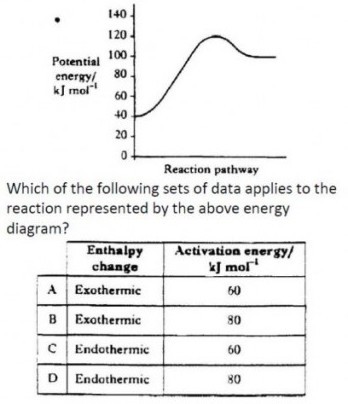
| In exothermic reactions, chemical potential energy is changed into heat energy, and released, causing the temperature to rise. As energy is released in this reaction, the enthalpy of the products (Hp) must be lower than the enthalpy of the reactants (Hr). ΔH must therefore have a negative value for an exothermic reaction. |
| In endothermic reactions, heat energy is taken in from the surroundings and changed into chemical potential energy, causing the temperature to fall. As energy is gained in this reaction, the enthalpy of the products (Hp) must be greater than the enthalpy of the reactants (Hr). ΔH must therefore have a positive value for an endothermic reaction.
The activation energy (for any reaction) is the difference between the reactant enthalpy and the high point on the reaction pathway. Activation energy is always positive, as the energy is taken in by the reactants.
|