  |
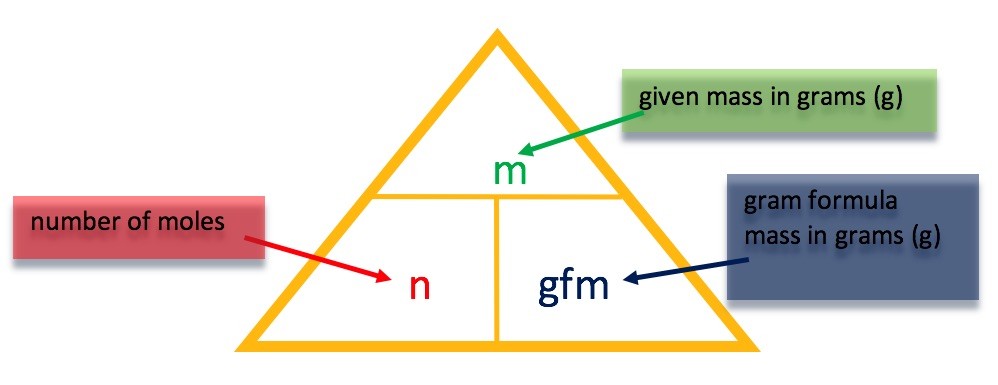
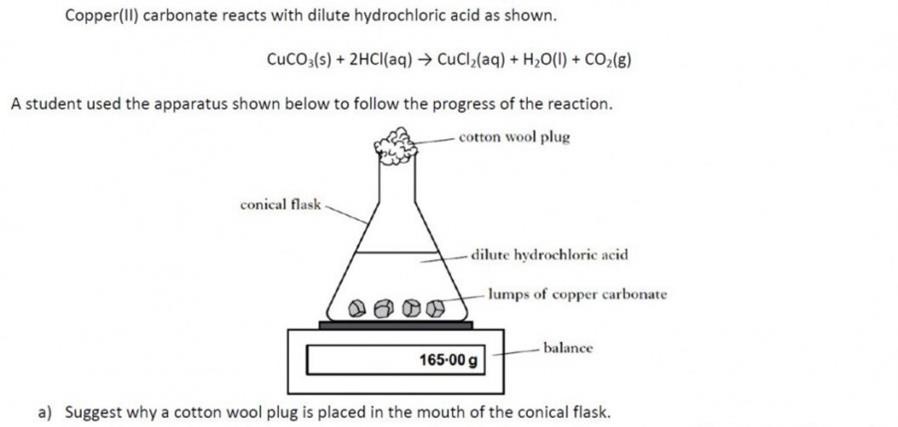
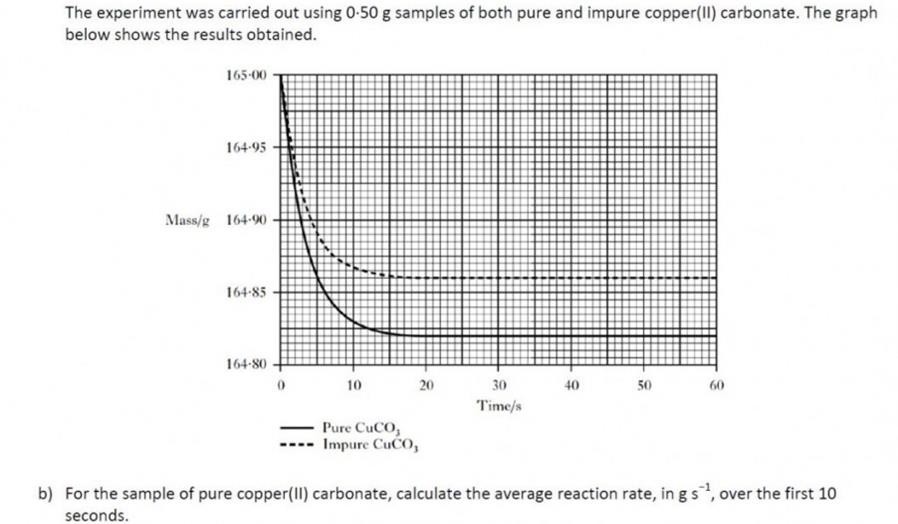
| At first this might seem a bit daunting. However, it can be assumed that only copper(II) carbonate has reacted in this experiment. As the copper (II) carbonate reacts, carbon dioxide is released, causing the fall in mass.From the mass of carbon dioxide, we can work out the number of moles of carbon dioxide we have lost.
If we know the molar relationship between the copper(II) carbonate and carbon dioxide – from the balanced equation, then we can work out the number of moles and hence the mass of copper(II) carbonate which must have originally been present. For help on molar ratios (this is covered in Unit 3) – click this link. |
Skip to content
Higher Chemistry Unit 1 Consolidation Exercises
Hyndland Chemistry Department