  |
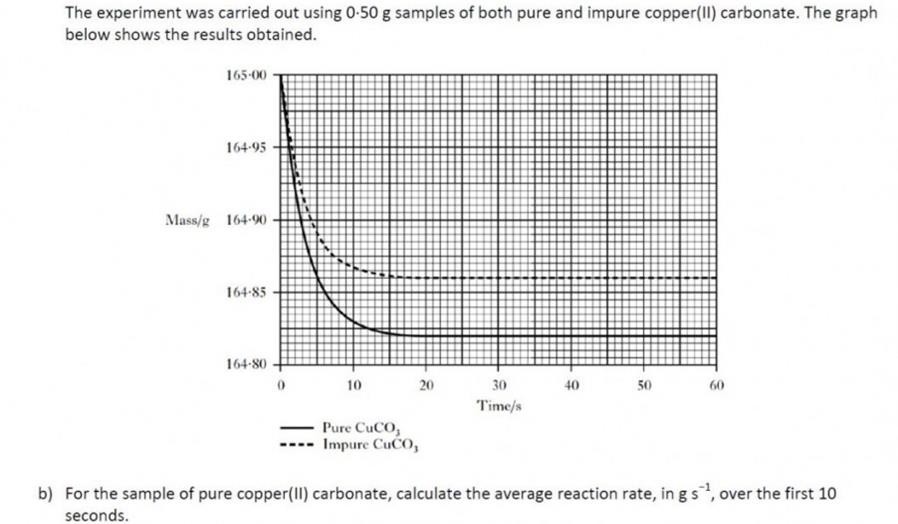
In this question, the term average can be confusing. We call it an average rate because the rate is changing as the reaction proceeds in the first 15s or so of the reaction, as shown by the curve of the line. ( A constant rate would produce a straight line).The average rate is simply calculated in these circumstance by examining the change in quantity (from the graph) and dividing that by the time taken.
You can find out more about rate calculations from this link. |
Skip to content
Higher Chemistry Unit 1 Consolidation Exercises
Hyndland Chemistry Department

