 |
Reaction Rate Revision Resources:
| Written Revision Questions | Multiple Choice Revision Questions |
 |
|
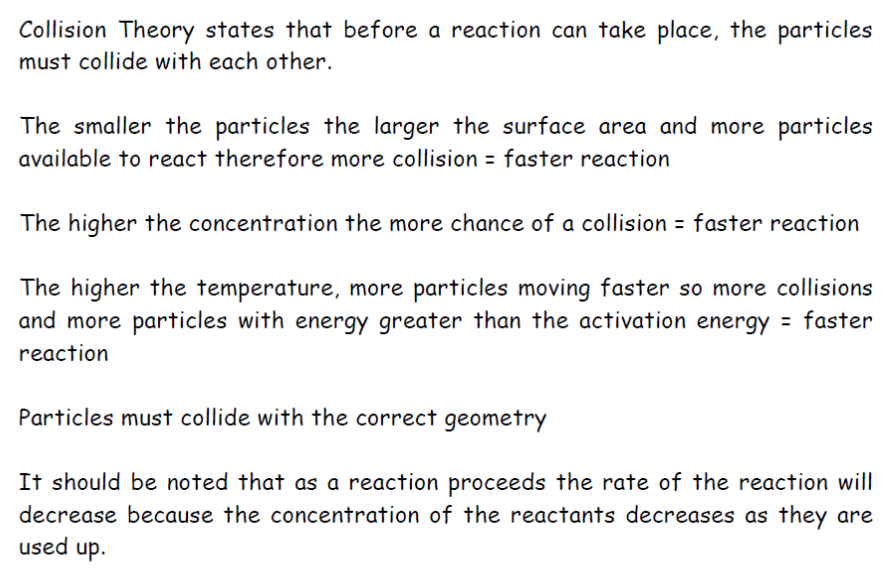
Factors affecting the rate of Reaction
Concentration of Reactants(applies to reactions involving solutions only) Pressure(applies to reactions involving gases only) Surface Area(applies to reactions involving solids) More collisions means more reactions. TemperatureThe higher the temperature, the faster the rate of reaction. Temperature measures the average kinetic energy of the reacting particles in the substance. The graph below shows the energy of each molecule in a sample at higher and lower temperatures. The molecules have different levels of kinetic energy. According to collision theory, molecules need to collide with sufficient energy before they can react. The minimum energy required to react is called the activation energy for the reaction. Examining the graph, only some of the particles will have sufficient enough kinetic energy to react, and those with lower kinetic energy will not be able to react. As the temperature increases, the proportion of particles with higher energy is also increased. If more molecules have sufficient energy to react, a greater of proportion of collisions will result in a successful reaction (fruitful collisions). |
Collision GeometryFor a collision to cause a reaction, moleules must collide with the correct orientation. This is best illustrated by two examples: In the reaction between ethene and hydrogen chloride, illustrated below, only if the hydrogen side of the H-Cl bond meets the carbon-carbon (double bond), will a reaction occur. If it collides with the ethene molecule in a different alignment no reaction will occur. |
 |
| In this second example two hydrogen atom react to form a hydrogen molecule. No bonds are broken, only formed. In this case, because hydrogen atoms are symmmetrical in 3 dimensions collision geometry is identical regardless of where the atoms approach each other, therefore in this case, collision geometry has no impact on reaction rate – this is an unusual case. |

|
| UC Davis Chemwiki |
Use of a CatalystA catalyst reduces the activation energy of a chemical reaction. It is also said to provide an alternative pathway for the reaction to occur. In doing so, catalysts speed up the rate of a chemical reaction. More information on the action of catalysts will be found in other section of Unit 1. |


