 |
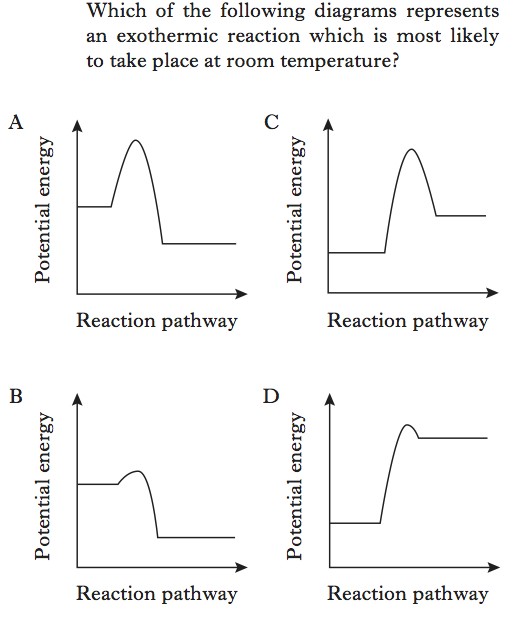
The key points you need to consider in this question are as follows:
- Chemical reactions require energy to occur.
- The Activation Energy (for any reaction) is the difference between the reactant enthalpy and the high point on the reaction pathway.
- The lower the Activation Energy, the less energy is required for a reaction to occur.
- The warmer the environment, the more energy there is available to allow a reaction to occur. That’s why heating a chemical reaction speeds it up.
These considerations will reduce you two possible options.
The following allows you to choose the correct answer.
- In exothermic reactions, chemical potential energy is changed into heat energy, and released. As energy is released the enthalpy of the products (Hp) must be lower than the enthalpy of the reactants (Hr). ΔH is negative for an exothermic reaction.
- In endothermic reactions, heat energy is taken in from the surroundings and changed into chemical potential energy. As energy is gained in this reaction, the enthalpy of the products (Hp) must be greater than the enthalpy of the reactants (Hr). ΔH is positive for an endothermic reaction.
|

