Click the QR code for MC Questions. Written questions, use your jotter, date & heading: ExN5 S4_3C Titration, in your jotter. |
 |
 |
| 1. | |
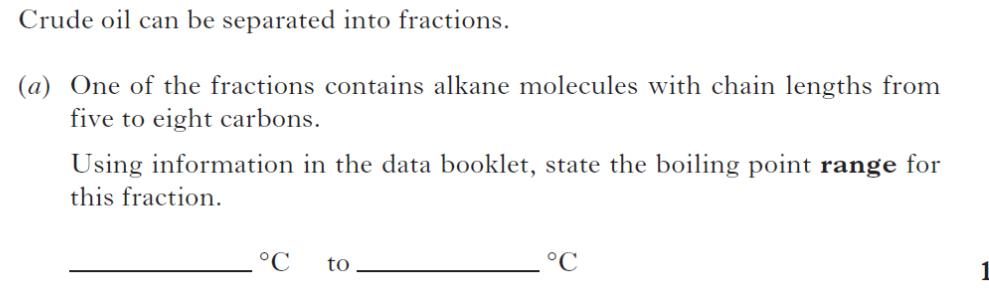 |
|
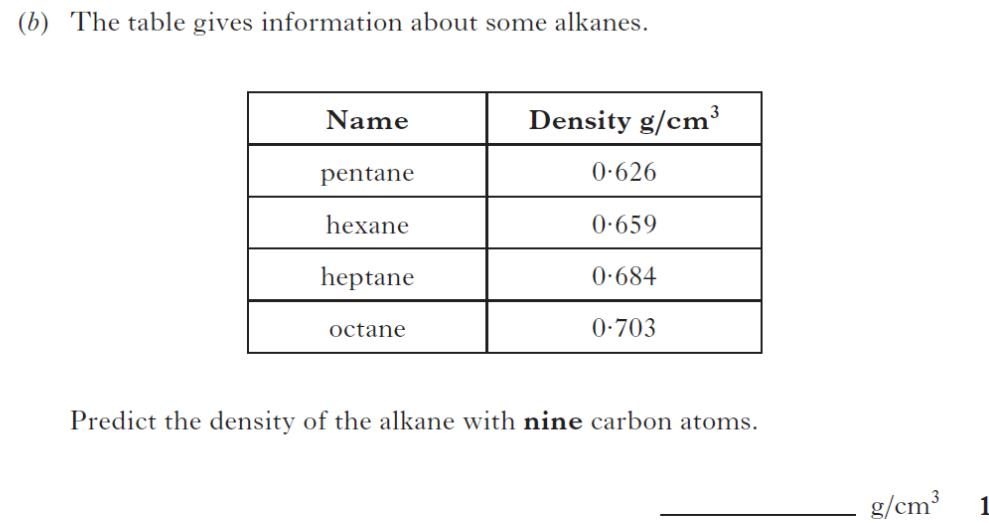 |
|
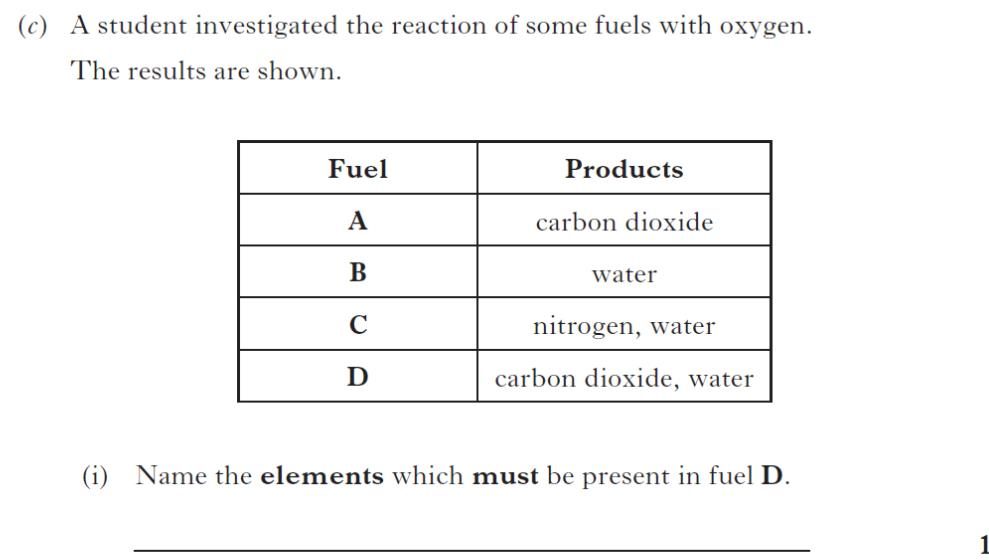 |
|
 |
|
| 2. | 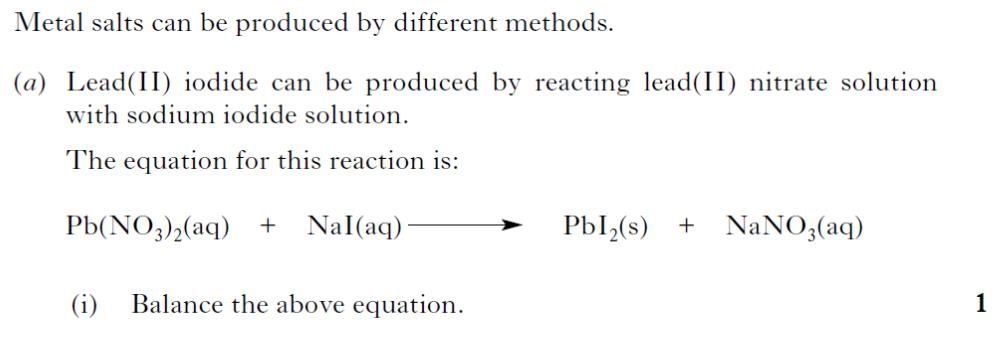 |
 |
|
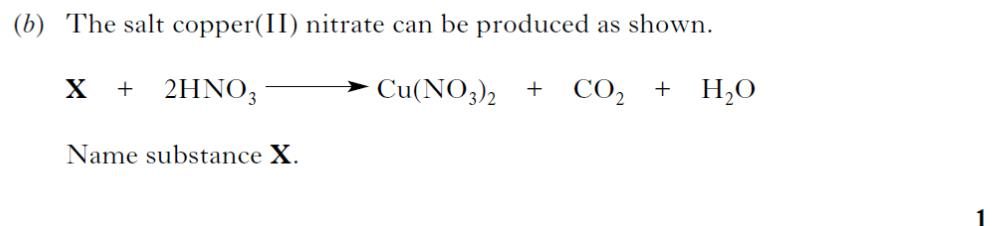 |
|
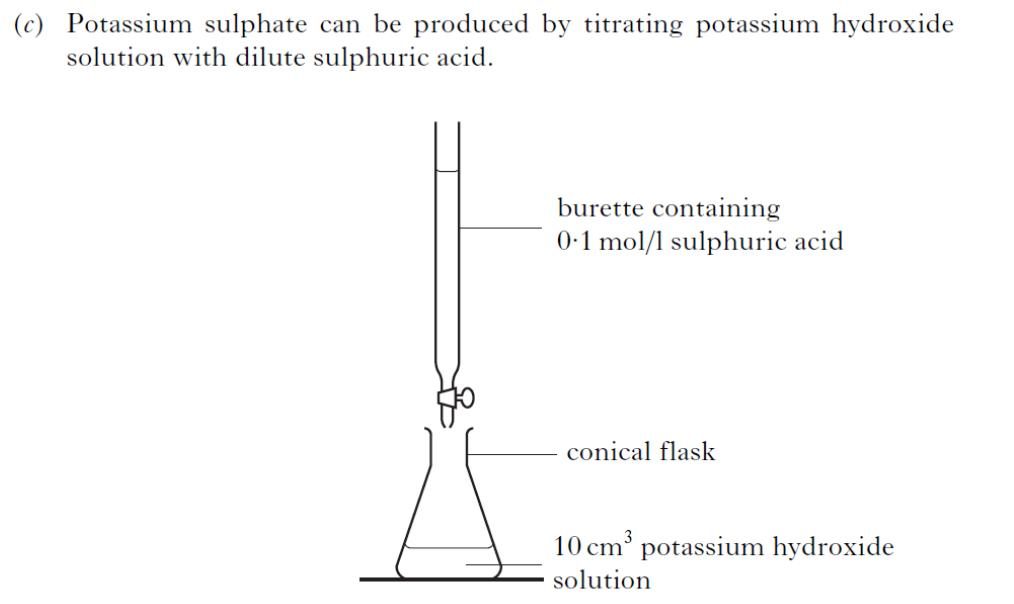 |
|
 |
|
 |
|
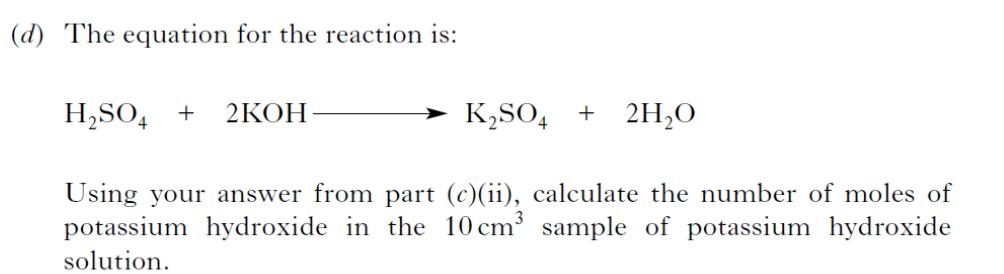 |
Question 3. Write the ionic formula for the following compounds:
| a) | lead (IV) fluoride | b) | calcium phosphide |
| c) | calcium phosphate | d) | beryllium oxide |
| e) | rubidium iodide | f) | vanadium (V) oxide |
| g) | iron (II) hydroxide | h) | copper (II) nitrate |
| i) | ammonium nitrate | j) | ammonium carbonate |
| k) | zinc (II) hydroxide | l) | copper (II) sulfate |

