Click the QR code for MC Questions. Written questions, use your jotter, date & heading: ExN5 S4_3A Titration. |
 |
 |
| 1. | |
 |
|
 |
|
| 2. | |
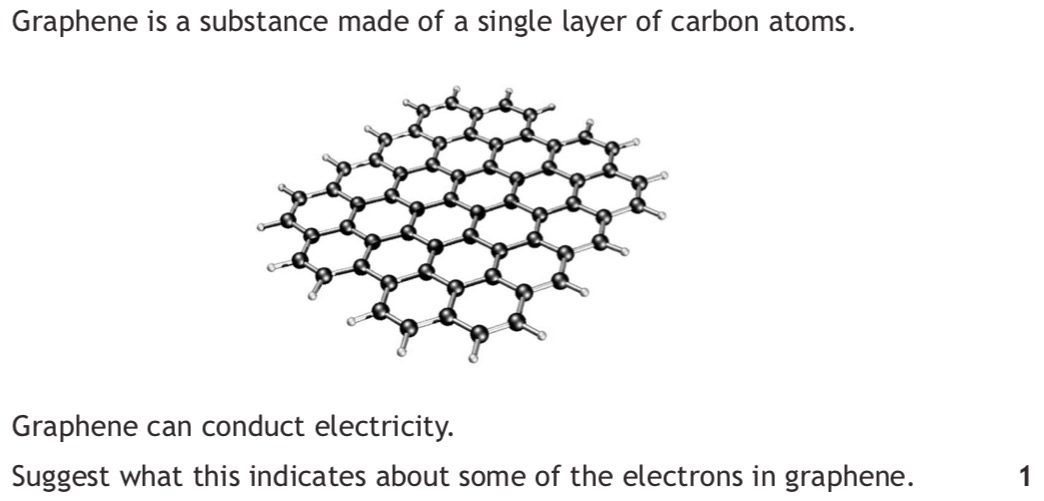 |
Question 3.
Calculate the concentration of the solutions made when dissolving:
| 4. | |
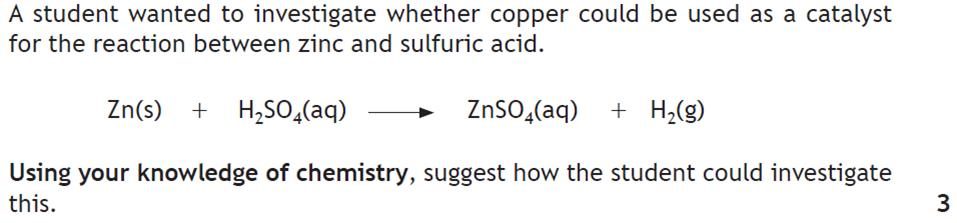 |
Hyndland Chemistry Department
Click the QR code for MC Questions. Written questions, use your jotter, date & heading: ExN5 S4_3A Titration. |
 |
 |
| 1. | |
 |
|
 |
|
| 2. | |
 |
Question 3.
Calculate the concentration of the solutions made when dissolving:
| 4. | |
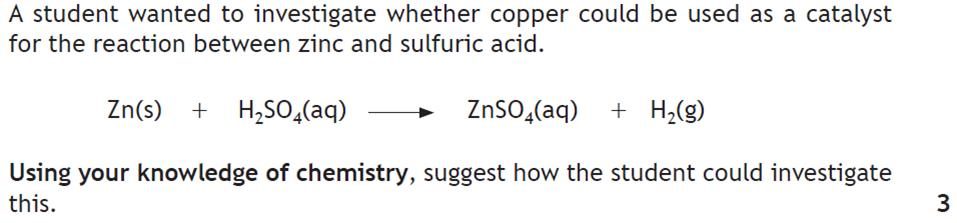 |