| Write the date in the margin of your homework jotter.
Write the title of this Exercise as a heading: Exercise N5 S4_1A Gram Formula Mass |
 |
| In these revision exercises you are asked to use the relationship between number of moles, gram formula mass and the mass of a substance to work out the answer. You should be familiar with the triangle formula and how to manipulate it. It is important you also appreciate that the units must be correct. |
 |
| 1. | 5. |
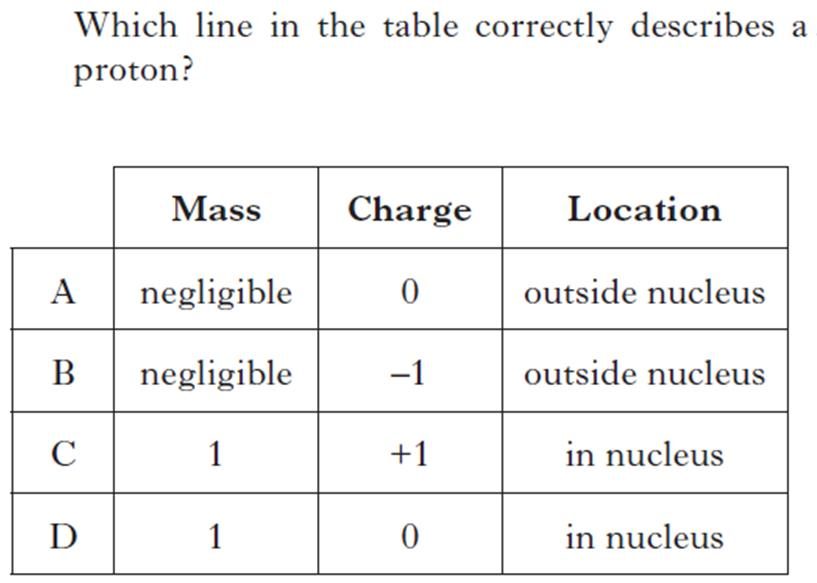  |
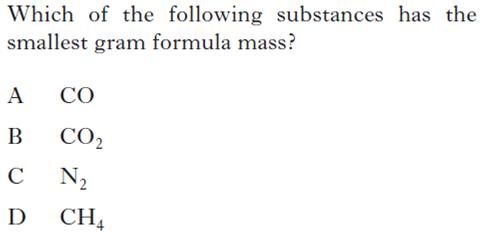  |
| 2. | 6. |
  |
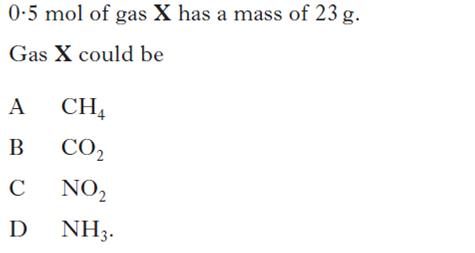  |
| 3. | 7. |
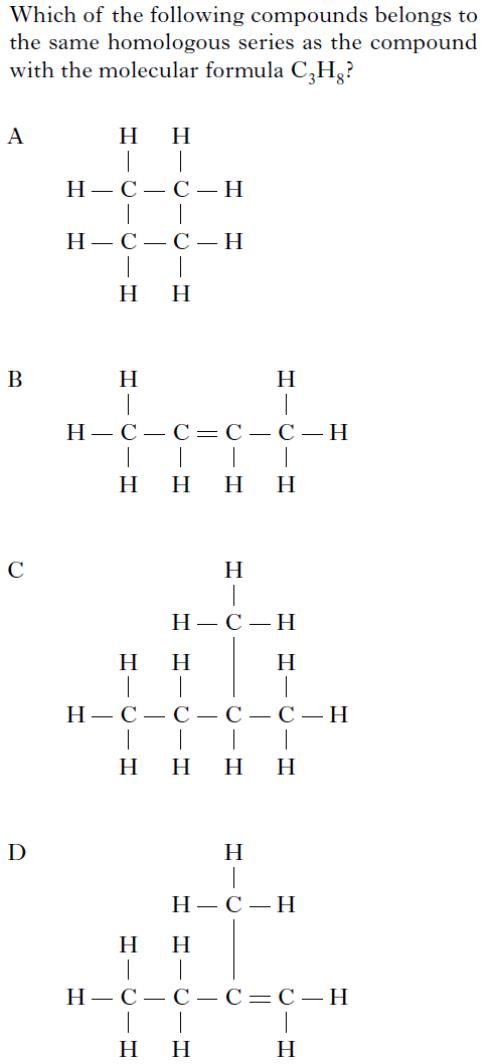  |
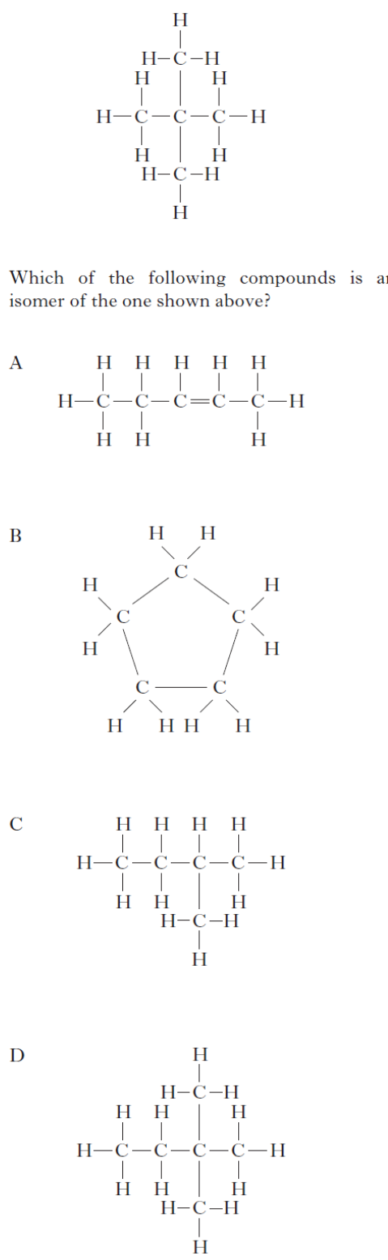  |
| 4. | 8. |
  |
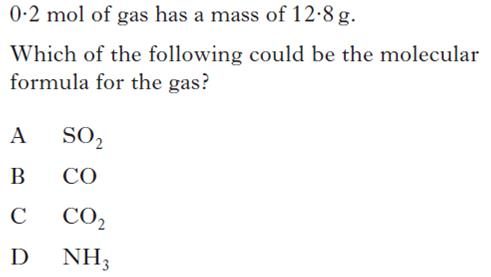  |
Question 9.
Calculate the mass of one mole of the following compounds:
| a) |  |
b) | 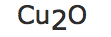 |
 |
 |
||
| c) | |
d) |  |
 |
 |
||
| e) | 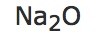 |
f) | 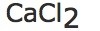 |
 |
 |
Question 10.
Find the mass of each of the following:
| a) | 0.2 moles of: | b) | 0.3 moles of: | ||
 |
|
 |
|
||
| c) | 0.25 moles of: | |
d) | 3 moles of: | |
 |
|
 |
|
||
| e) | 10 moles of: | f) | 0.4 moles of: | ||
 |
|
 |
|
Question 11.
Find the mass of each of the following:
| a) | 2 moles of: | b) | 3 moles of: | ||
 |
|
 |
|
||
| c) | 25 moles of: | 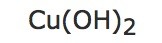 |
d) | 0.3 moles of: | 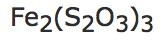 |
 |
|
 |
|
||
| e) | 1.3 moles of: | f) | 2.4 moles of: | ||
 |
|
 |
|
Question 12.
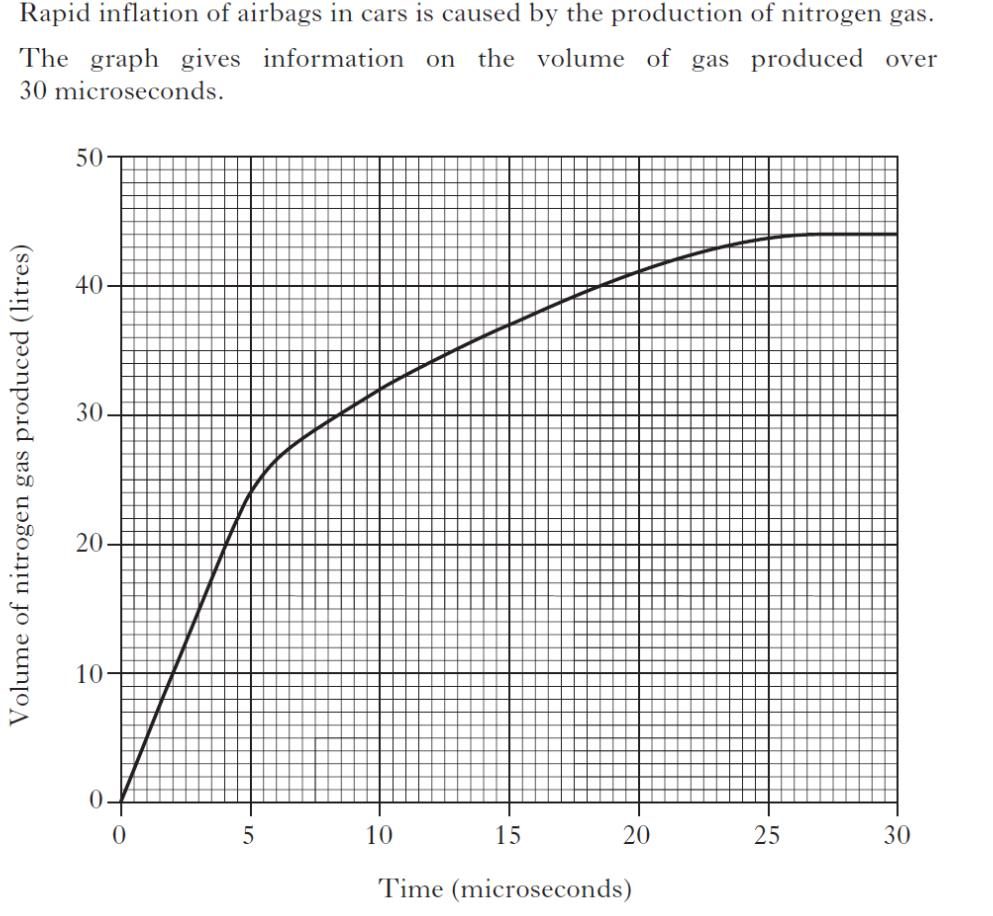 |
|
| a) | |
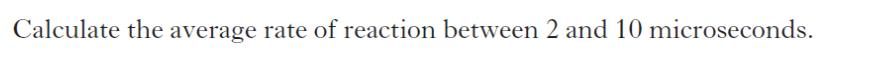 |
|
 |
|
| b) | |
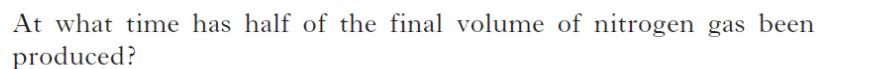 |
|
 |
|
| c) | |
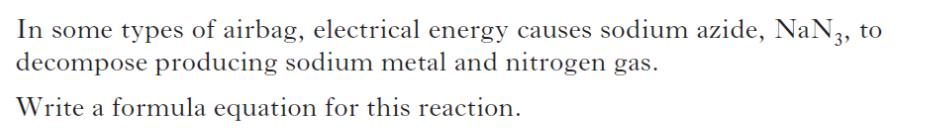 |
|
 |
|
| d) | |
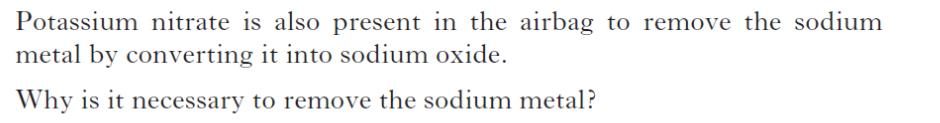 |
|
 |
Question 13.
 |
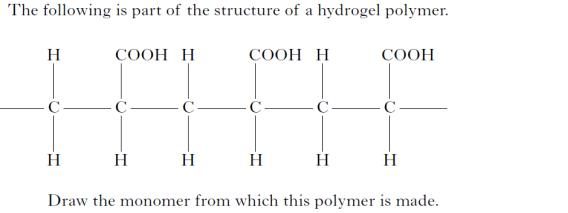 |
 |

