Click the QR code for MC Questions. Written questions, use your jotter, date & heading: ExN5 S4_3B Titration. |
 |
 |
| 1. | |
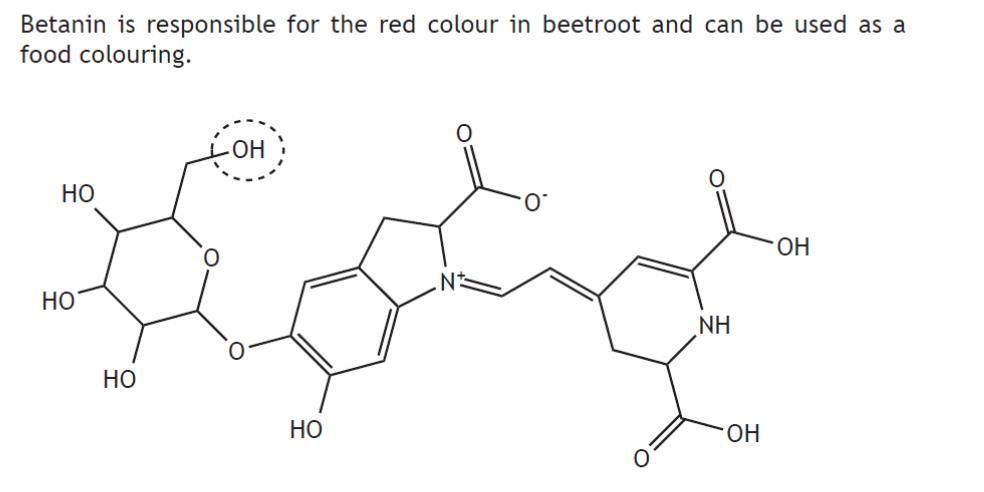 |
|
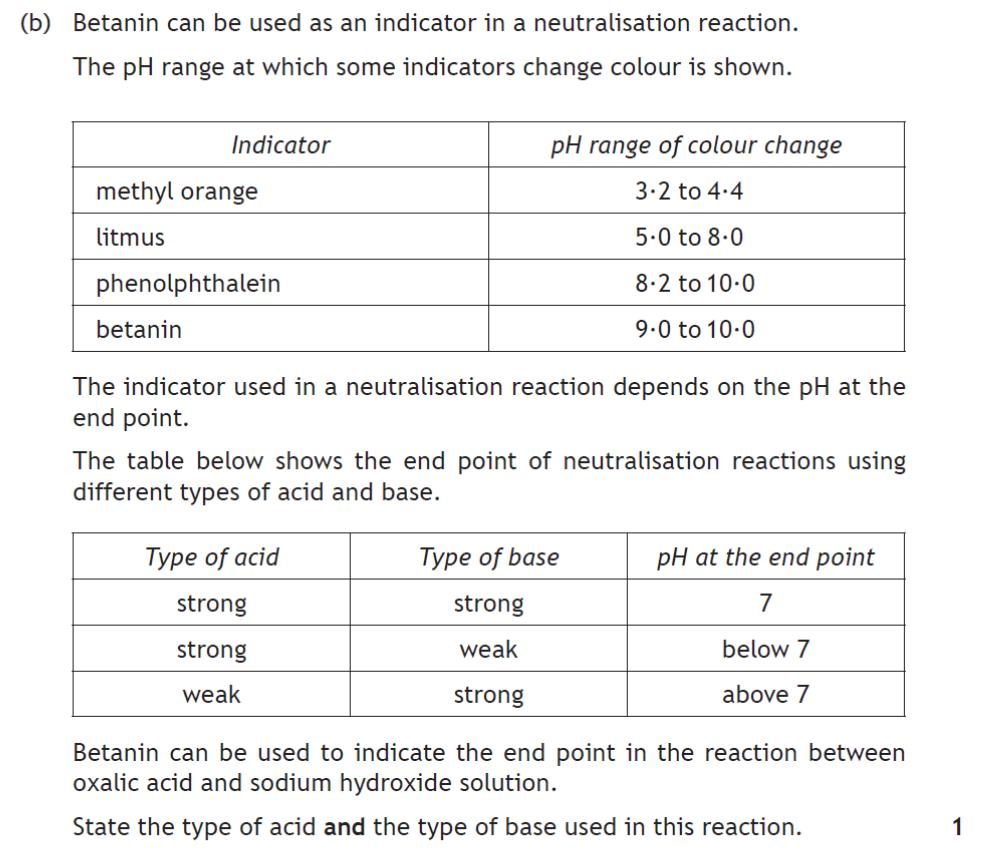 |
|
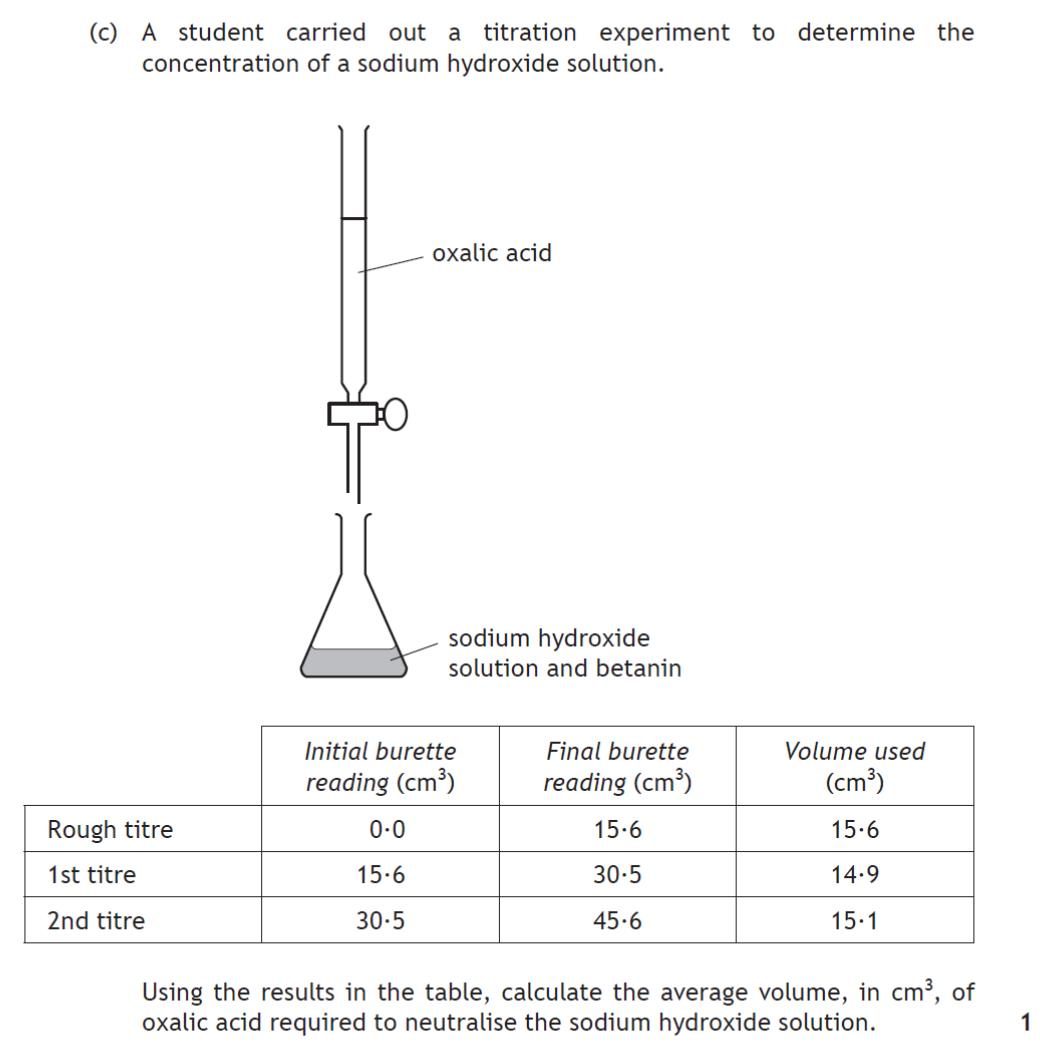 |
|
 |
| 2. | |
 |
Question 3.
Calculate the number of grams of substance required to make the following solutions
| a) | 100 cm3 of Mg(OH)2, concentration 2 mol l-1 |
| b) | 50 cm3 of KOH, concentration 0.5 mol l-1 |
| c) | 25 cm3 of caesium nitrate, concentration 0.2 mol l-1 |
| d) | 250 cm3 of ammonium sulfate, concentration 1 mol l-1 |
| e) | 200 cm3 of calcium phosphate, concentration 0.25 mol l-1 |
| f) | 50 cm3 of aluminium phosphate, concentration 0.5 mol l-1 |
Question 4.
Calculate the concentration of each of the following solutions.
| a) | 25g of CaCO3 dissolved to make 0.5 litres of solution |
| b) | 20.5 g of Ca(NO3)2 dissolved to make 400 cm3 of solution |
| c) | 1.325g of Na2CO3 dissolved to make 100 cm3 of solution |
| d) | 5.8g of potassium sulfate dissolved to make 250 cm3 of solution |
| e) | 9.4g of copper (II) nitrate dissolved to make 500 cm3 of solution |
| f) | 4g of iron(III) sulfate dissolved to make 50 cm3 of solution |

