All substances are made of atoms. There are around 100 different types of atom, listed in the period table. All atoms have the same basic structure, consisting of a nucleus containing protons and neutrons, surrounded by electrons held in various shells or levels.
|
The nucleus, because it contains the positively charged protons is positively charged. The neutrons in the nucleus have no charge, and help to hold the protons together (like charges repel and so neutrons allow the protons to be held tightly together despite their charge repulsion) – acting like a sort of glue. The electrons are negatively charged and occupy their shells according to certain rules. Each shell (1,2,3,4 etc.) can only hold a maximum number of electrons. |
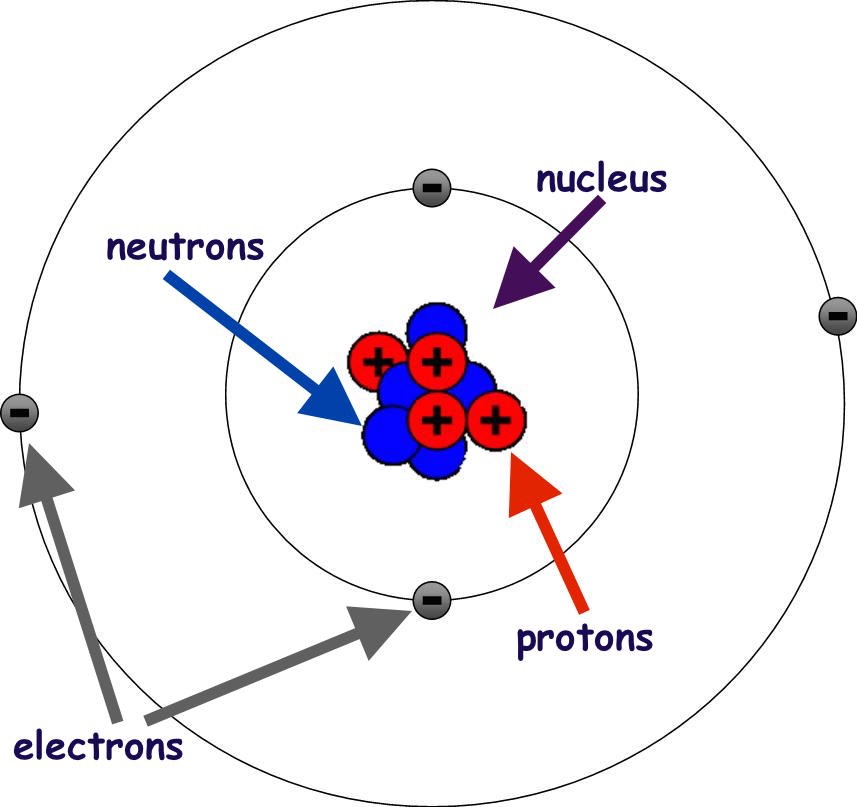 |
|
Shell 1 holds 2 electrons max. Electrons arrangements are written in a shorthand format. |
chemwiki.ucdavis.edu |

