 At National 5 level you are expected to be able to name chemical compounds and understand what the name of a chemical compound tells you.
At National 5 level you are expected to be able to name chemical compounds and understand what the name of a chemical compound tells you.
Compounds are named based on the elements they contain. Convention suggests that metal elements should be given first in a name e.g. sodium chloride, not chlorine sodium.
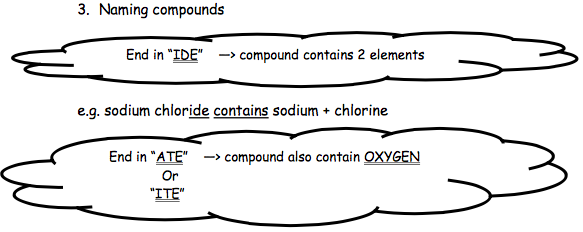
You will encounter three endings to a chemical’s name: IDE, ITE and ATE. Each of the endings given you important information about the elements contained in the compound
| Ending | Explanation | Examples |
| IDE | The ending IDE tells you that only those elements named in the compound are present | calcium oxide contains:calcium and oxygen only.calcium hydroxide contains:
calcium, hydrogen and oxygen only. |
| ATE | The ending ATE tells you that in addition to those elements named in the compound, oxygen is also present. | sodium sulfate contains:sodium, sulfur and oxygen.potassium nitrate:
contains potassium, nitrogen and oxygen |
| ITE | The ending ITE tells you that in addition to those elements named in the compound, oxygen is also present. The difference between ATE and ITE is that there is less oxygen present, but this is not important at National 5 level. | iron sulfite:contains iron, sulfur and oxygen. |

