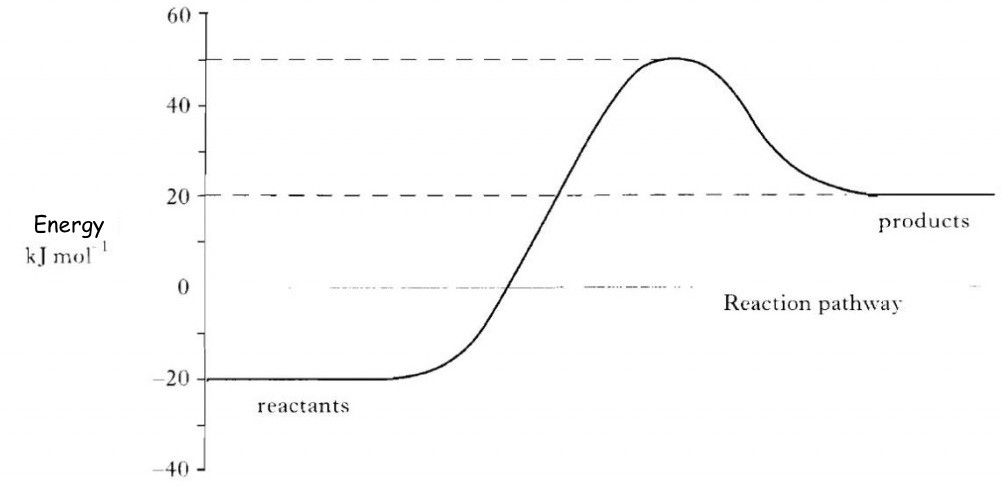
| In an Exothermic reaction, energy is released. This can be detected by an increase in the temperature of the surroundings.Whilst many reactions are endothermic those chemical reactions that heat us, e.g. the burning (combustion) of fuels and the combustion of petrol in a car engine are two notable exothermic reactions.Hand warmers are often powered by exothermic chemical reactions.
The change in energy can be shown in an energy graph. It shows that the energy of the reactants is higher than that of the products, indicating that energy is lost to the environment during this type of reaction. |
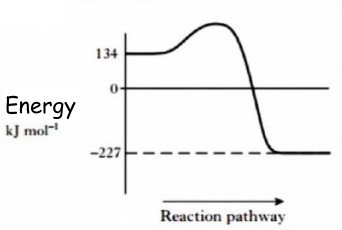 |
| In an Endothermic reaction, energy is taken in during the reaction. This can be detected by a decrease in the temperature of the surroundings.There are fewer well known endothermic reactions, but some types of freeze packs, used to treat strains in sports are powered by endothermic reactions.
The change in energy can be shown in an energy graph. It shows that the energy of the reactants is lower than that of the products, indicating that energy has been gained from the environment during this type of reaction. |
 |