Click the QR code for MC Questions. Written questions, use your jotter, date & heading: ExN5 S3_6C Bonding & Structure. |
 |
 |
- Write the ionic formula for the following compounds:
| a) | calcium oxide | b) | sodium bromide |
| c) | copper (I) bromide | d) | copper (I) sulfide |
| e) | nickel (II) fluoride | f) | vanadium (V) oxide |
| g) | barium iodide | h) | iron (III) sulphide |
| i) | iron (III) oxide | j) | tin (IV) oxide |
| k) | magnesium oxide | l) | zinc (I) sulfide |
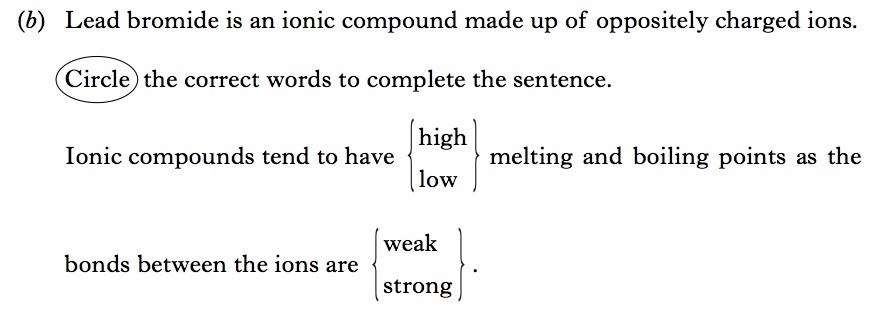
| 2. | |
  |
| 3. | Click the QR code to access the next questions on bonding in compounds. |  |
| 4. | Write chemical formula for the following molecules: |
| (a) | phosphorus iodide | (b) | carbon fluoride |
| (c) | silicon bromide | (d) | silicon tetrachloride |
| (e) | phosphorus oxide | (f) | hydrogen sulfide |
| (g) | sulfur iodide | (h) | oxygen |
| 5. | Draw diagrams, showing outer electrons, to show how the following molecules form covalent bonds. |
| (a) | fluorine | (b) | oxygen |
| (c) | nitrogen | (d) | hydrogen fluoride |
| (e) | nitrogen hydride | (f) | carbon hydride |

