| 1. | 5. |
 |
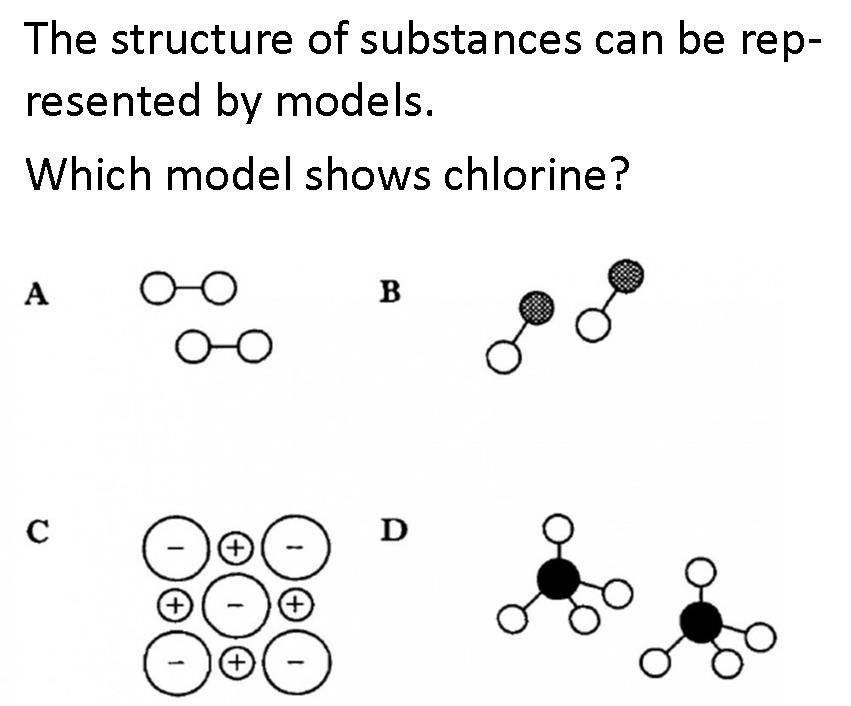 |
| 2. | 6. |
 |
 |
| 3. | 7. |
 |
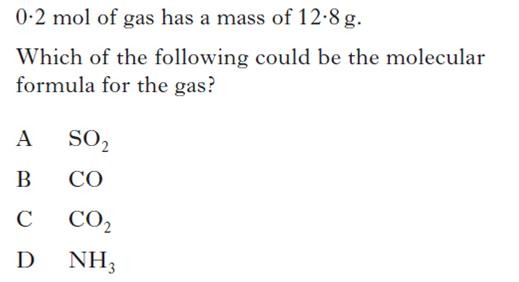 |
| 4. | 8. |
 |
 |
For each of the questions which follow show your working
| A marking guide for this Homework is available (password required). |
Hyndland Chemistry Department
| 1. | 5. |
 |
 |
| 2. | 6. |
 |
 |
| 3. | 7. |
 |
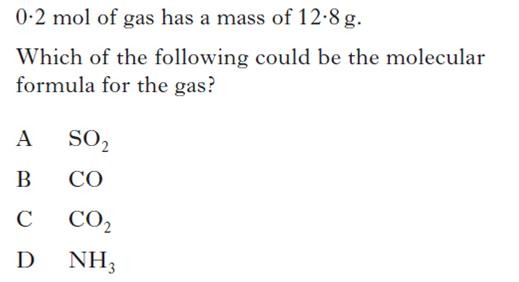 |
| 4. | 8. |
 |
 |
For each of the questions which follow show your working
| A marking guide for this Homework is available (password required). |