| In atom, electrons are held in shells (also called orbits or energy levels) around the nucleus. Each shell can only hold a specified number of electrons, and for elements 1 to 20 (all that is necessary for National 5), the first 4 shells are used. |  |
| The maximum number of electrons which each in shell can hold is: | montessorimuddle.org |
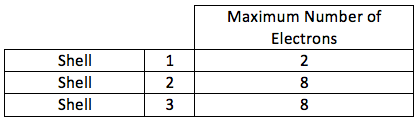 |
|
| The increase in row number for the periodic table, reflects an increase in the number of shells which contain electrons. As we move across the periodic table, the number of electrons in the outermost is increased by 1 each time.In the next row of the periodic table, the third shell becomes occupied.Electrons find the lowest number shell. So the first shell is occupied by all elements, the second shell then gets filled as we move across the period table. |
Examples:
| The element oxygen, atomic number 8 |
| 8 protons, and 8 electrons |
| electron arrengement 2,6 |
– 2 electrons in the first shell (filled)
– 6 electrons in the second shell (needs a further 2 electrons before it is filled)
| The element sodium, atomic number 11 |
| 11 protons and 11 electrons |
| electron arrangement 2,8,1 |
– 2 electrons in the innermost shell (filled)
– 8 electrons in the next shell (filled)
– 1 electron in the third, outermost shell.

