| MCQ | Answer | Area of Chemistry |
| 1. | D | [Properties of Materials] |
| 2. | B | [Balancing Equations] |
| 3. | C | [Naming hydrocarbons] |
| 4. | B | [Naming Compounds] |
| 5. | A | [Atomic Structure – Ions] |
| 6. | A | [Gram Formula Mass] |
| 7. | C | [Nuclide Notation – Problem Solving] |
| 8. | C | [Diatomic Elements] |
| 9. | ||
| a) | (NH4)2CO3 | |
| b) | Al2(SO4)3 | |
| c) | CaSO4 | |
| d) | Mg(OH)2 | |
| e) | NH4NO3 | |
| f) | Ca(NO3)2 | |
| [Group Ion Formulae] |
| 10. | |
| a) | K+Cl– |
| b) | Mg2+(Br–)2 |
| c) | Ca2+S2- |
| d) | Ca2+(H–)2 |
| e) | (Na+)2O2- |
| f) | Li+Cl– |
| g) | Pb4+(O2-)2 |
| h) | Al3+(Br–)3 |
| i) | Cs+Cl– |
| [Ionic Formulae] |
| -11- | |
| a) | 11g |
 |
|
| b) | 19.1g |
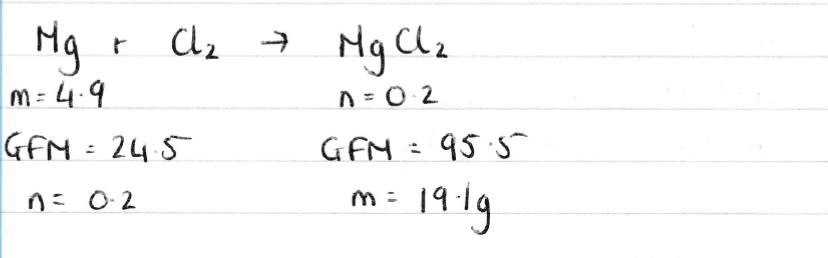 |
|
| c) | 11.7g |
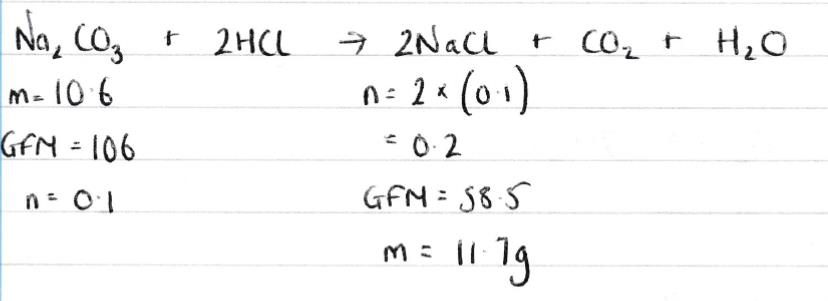 |
|


