| 1. | |
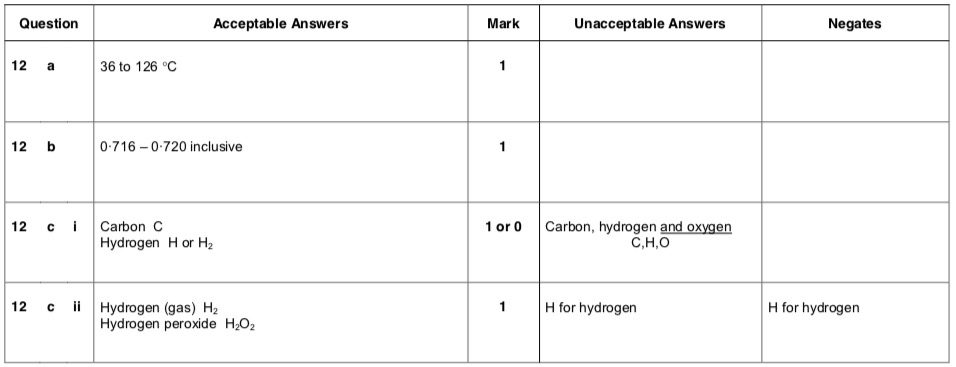 |
|
| 2. | |
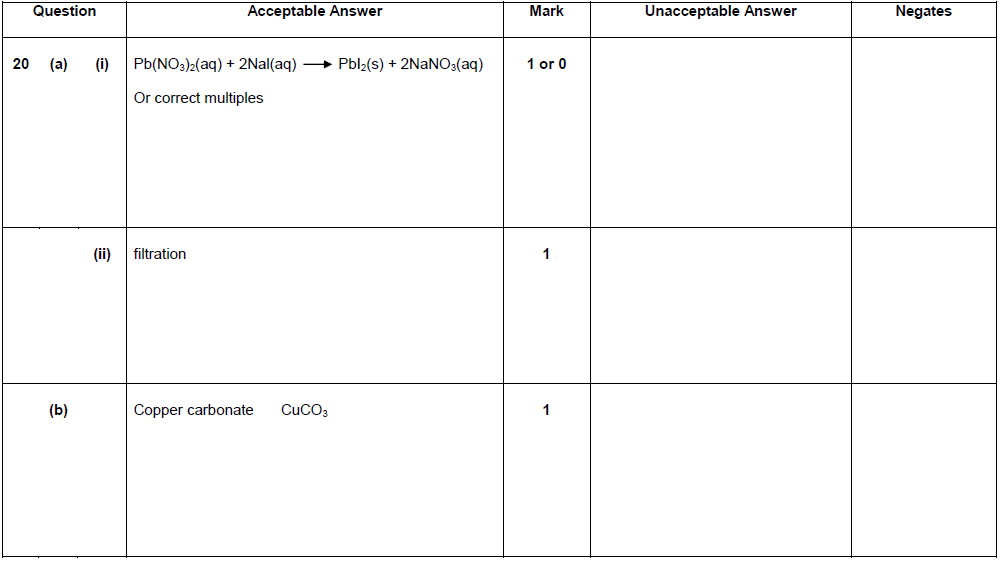 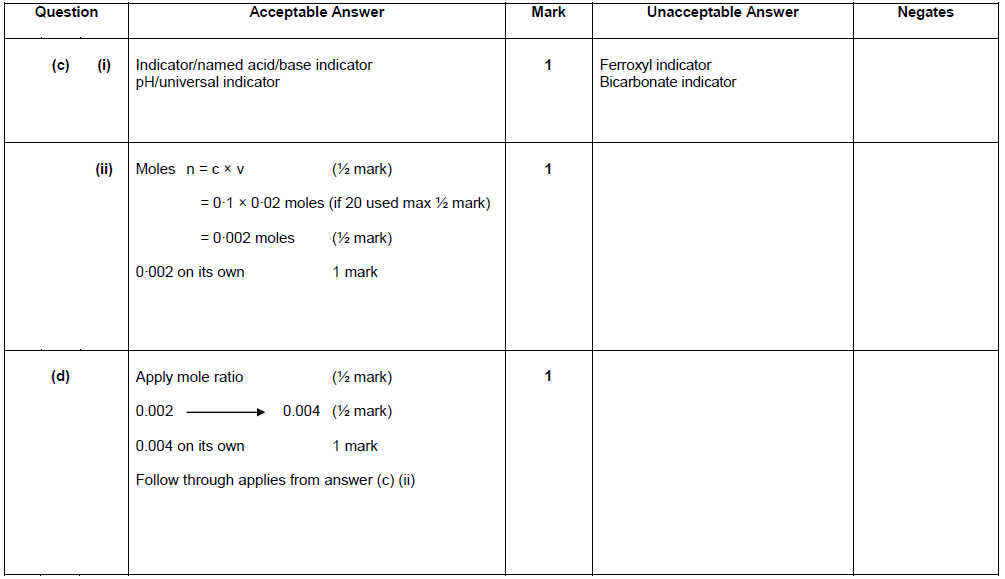 |
| 3. | ||
| a) | Pb4+(F–)4 | |
| b) | (Ca2+)3(P3-)2 | |
| c) | (Ca2+)3(PO43-)2 | |
| d) | Be2+O2- | |
| e) | Rb+I– | |
| f) | (V5+)2(O2-)5 | |
| g) | Fe2+(OH–)2 | |
| h) | Cu2+(NO3–)2 | |
| i) | NH4+NO3– | |
| j) | (NH4+)2CO32- | |
| k) | Zn2+(OH–)2 | |
| l) | Cu2+SO42- |
National 5 Chemistry Homework Answers
Hyndland Science Faculty
| 1. | |
 |
|
| 2. | |
  |
| 3. | ||
| a) | Pb4+(F–)4 | |
| b) | (Ca2+)3(P3-)2 | |
| c) | (Ca2+)3(PO43-)2 | |
| d) | Be2+O2- | |
| e) | Rb+I– | |
| f) | (V5+)2(O2-)5 | |
| g) | Fe2+(OH–)2 | |
| h) | Cu2+(NO3–)2 | |
| i) | NH4+NO3– | |
| j) | (NH4+)2CO32- | |
| k) | Zn2+(OH–)2 | |
| l) | Cu2+SO42- |